Modulation of Carnitine Palmitoyl Transferase 1b Expression and Activity in Muscle Pathophysiology in Osteoarthritis and Osteoporosis
- PMID: 39456222
- PMCID: PMC11505991
- DOI: 10.3390/biom14101289
Modulation of Carnitine Palmitoyl Transferase 1b Expression and Activity in Muscle Pathophysiology in Osteoarthritis and Osteoporosis
Abstract
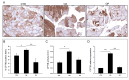
In the pathophysiology of osteoarthritis and osteoporosis, articular cartilage and bone represent the target tissues, respectively, but muscle is also involved. Since many changes in energy metabolism occur in muscle with aging, the aim of the present work was to investigate the involvement of carnitine palmitoyl transferase 1b (Cpt1b) in the muscle pathophysiology of the two diseases. Healthy subjects (CTR, n = 5), osteoarthritic (OA, n = 10), and osteoporotic (OP, n = 10) patients were enrolled. Gene expression analysis conducted on muscle and myoblasts showed up-regulation of CPT1B in OA patients; this result was confirmed by immunohistochemical and immunofluorescence analyses and enzyme activity assay, which showed increased Cpt1b activity in OA muscle. In addition, CPT1B expression resulted down-regulated in cultured OP myoblasts. Given the potential involvement of Cpt1b in the modulation of oxidative stress, we investigated ROS levels, which were found to be lower in OA myoblasts, and gene expression of nicotinamide adenine dinucleotide phosphate hydrogen oxidase 4 (Nox4), which resulted up-regulated in OA cells. Finally, the immunofluorescence of BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3) showed a decreased expression in OP myoblasts, with respect to CTR and OA. Contextually, through an ultrastructural analysis conducted by Transmission Electron Microscopy (TEM), the presence of aberrant mitochondria was observed in OP muscle. This study highlights the potential role of Cpt1b in the regulation of muscle homeostasis in both osteoarthritis and osteoporosis, allowing for the expansion of the current knowledge of what are the molecular biological pathways involved in the regulation of muscle physiology in both diseases.
Keywords: energy metabolism; mitochondria biogenesis; muscle atrophy; myoblast regeneration; osteoarthritis; osteoporosis; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

