The Ambivalence of Post COVID-19 Vaccination Responses in Humans
- PMID: 39456253
- PMCID: PMC11506738
- DOI: 10.3390/biom14101320
The Ambivalence of Post COVID-19 Vaccination Responses in Humans
Abstract
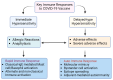
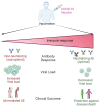
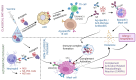
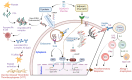
The Coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has prompted a massive global vaccination campaign, leading to the rapid development and deployment of several vaccines. Various COVID-19 vaccines are under different phases of clinical trials and include the whole virus or its parts like DNA, mRNA, or protein subunits administered directly or through vectors. Beginning in 2020, a few mRNA (Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273) and adenovirus-based (AstraZeneca ChAdOx1-S and the Janssen Ad26.COV2.S) vaccines were recommended by WHO for emergency use before the completion of the phase 3 and 4 trials. These vaccines were mostly administered in two or three doses at a defined frequency between the two doses. While these vaccines, mainly based on viral nucleic acids or protein conferred protection against the progression of SARS-CoV-2 infection into severe COVID-19, and prevented death due to the disease, their use has also been accompanied by a plethora of side effects. Common side effects include localized reactions such as pain at the injection site, as well as systemic reactions like fever, fatigue, and headache. These symptoms are generally mild to moderate and resolve within a few days. However, rare but more serious side effects have been reported, including allergic reactions such as anaphylaxis and, in some cases, myocarditis or pericarditis, particularly in younger males. Ongoing surveillance and research efforts continue to refine the understanding of these adverse effects, providing critical insights into the risk-benefit profile of COVID-19 vaccines. Nonetheless, the overall safety profile supports the continued use of these vaccines in combating the pandemic, with regulatory agencies and health organizations emphasizing the importance of vaccination in preventing COVID-19's severe outcomes. In this review, we describe different types of COVID-19 vaccines and summarize various adverse effects due to autoimmune and inflammatory response(s) manifesting predominantly as cardiac, hematological, neurological, and psychological dysfunctions. The incidence, clinical presentation, risk factors, diagnosis, and management of different adverse effects and possible mechanisms contributing to these effects are discussed. The review highlights the potential ambivalence of human response post-COVID-19 vaccination and necessitates the need to mitigate the adverse side effects.
Keywords: Bell’s palsy; Guillain–Barré syndrome; adverse effects; allergy; autoimmunity; immune activation; long COVID; myocarditis; proinflammatory response; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO WHO COVID-19 Dashboard. [(accessed on 15 September 2024)]. Available online: https://data.who.int/dashboards/covid19/cases?n=c.
-
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Features, Evaluation, and Treatment of Coronavirus (COVID-19) - PubMed
-
- da Rosa Mesquita R., Francelino Silva Junior L.C., Santos Santana F.M., Farias de Oliveira T., Campos Alcantara R., Monteiro Arnozo G., Rodrigues da Silva Filho E., Galdino Dos Santos A.G., Oliveira da Cunha E.J., Salgueiro de Aquino S.H., et al. Clinical manifestations of COVID-19 in the general population: Systematic review. Wien. Klin. Wochenschr. 2021;133:377–382. doi: 10.1007/s00508-020-01760-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

