Pulling Forces Differentially Affect Refolding Pathways Due to Entangled Misfolded States in SARS-CoV-1 and SARS-CoV-2 Receptor Binding Domain
- PMID: 39456260
- PMCID: PMC11505858
- DOI: 10.3390/biom14101327
Pulling Forces Differentially Affect Refolding Pathways Due to Entangled Misfolded States in SARS-CoV-1 and SARS-CoV-2 Receptor Binding Domain
Abstract
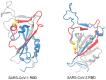
Single-molecule force spectroscopy (SMFS) experiments can monitor protein refolding by applying a small force of a few piconewtons (pN) and slowing down the folding process. Bell theory predicts that in the narrow force regime where refolding can occur, the folding time should increase exponentially with increased external force. In this work, using coarse-grained molecular dynamics simulations, we compared the refolding pathways of SARS-CoV-1 RBD and SARS-CoV-2 RBD (RBD refers to the receptor binding domain) starting from unfolded conformations with and without a force applied to the protein termini. For SARS-CoV-2 RBD, the number of trajectories that fold is significantly reduced with the application of a 5 pN force, indicating that, qualitatively consistent with Bell theory, refolding is slowed down when a pulling force is applied to the termini. In contrast, the refolding times of SARS-CoV-1 RBD do not change meaningfully when a force of 5 pN is applied. How this lack of a Bell response could arise at the molecular level is unknown. Analysis of the entanglement changes of the folded conformations revealed that in the case of SARS-CoV-1 RBD, an external force minimizes misfolding into kinetically trapped states, thereby promoting efficient folding and offsetting any potential slowdown due to the external force. These misfolded states contain non-native entanglements that do not exist in the native state of either SARS-CoV-1-RBD or SARS-CoV-2-RBD. These results indicate that non-Bell behavior can arise from this class of misfolding and, hence, may be a means of experimentally detecting these elusive, theoretically predicted states.
Keywords: SARS-CoV-1 RBD; SARS-CoV-2 RBD; folding pathways; lasso entanglement; protein folding; quenched force.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





Similar articles
-
SARS-CoV-2 omicron RBD forms a weaker binding affinity to hACE2 compared to Delta RBD in in-silico studies.J Biomol Struct Dyn. 2024 May;42(8):4087-4096. doi: 10.1080/07391102.2023.2222827. Epub 2023 Jun 22. J Biomol Struct Dyn. 2024. PMID: 37345564
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Influence of Cholesterol on the Insertion and Interaction of SARS-CoV-2 Proteins with Lipid Membranes.ACS Appl Bio Mater. 2025 Jun 16;8(6):5380-5394. doi: 10.1021/acsabm.5c00776. Epub 2025 Jun 6. ACS Appl Bio Mater. 2025. PMID: 40474679 Free PMC article.
-
Workplace interventions to reduce the risk of SARS-CoV-2 infection outside of healthcare settings.Cochrane Database Syst Rev. 2022 May 6;5(5):CD015112. doi: 10.1002/14651858.CD015112.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD015112. doi: 10.1002/14651858.CD015112.pub3. PMID: 35514111 Free PMC article. Updated.
-
Limited Variation between SARS-CoV-2-Infected Individuals in Domain Specificity and Relative Potency of the Antibody Response against the Spike Glycoprotein.Microbiol Spectr. 2022 Feb 23;10(1):e0267621. doi: 10.1128/spectrum.02676-21. Epub 2022 Jan 26. Microbiol Spectr. 2022. PMID: 35080430 Free PMC article.
References
-
- Baiesi M., Orlandini E., Seno F., Trovato A. Exploring the correlation between the folding rates of proteins and the entanglement of their native states. J. Phys. A Math. Theor. 2017;50:504001. doi: 10.1088/1751-8121/aa97e7. - DOI
-
- Nissley D.A., Jiang Y., Trovato F., Sitarik I., Narayan K.B., To P., Xia Y., Fried S.D., O’brien E.P. Universal protein misfolding intermediates can bypass the proteostasis network and remain soluble and less functional. Nat. Commun. 2022;13:3081. doi: 10.1038/s41467-022-30548-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

