Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review
- PMID: 39456412
- PMCID: PMC11505308
- DOI: 10.3390/antiox13101158
Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review
Abstract
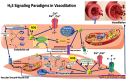
Hydrogen sulfide (H2S), a gas traditionally considered toxic, is now recognized as a vital endogenous signaling molecule with a complex physiology. This comprehensive study encompasses a systematic literature review that explores the intricate mechanisms underlying H2S-induced vasodilation. The vasodilatory effects of H2S are primarily mediated by activating ATP-sensitive potassium (K_ATP) channels, leading to membrane hyperpolarization and subsequent relaxation of vascular smooth muscle cells (VSMCs). Additionally, H2S inhibits L-type calcium channels, reducing calcium influx and diminishing VSMC contraction. Beyond ion channel modulation, H2S profoundly impacts cyclic nucleotide signaling pathways. It stimulates soluble guanylyl cyclase (sGC), increasing the production of cyclic guanosine monophosphate (cGMP). Elevated cGMP levels activate protein kinase G (PKG), which phosphorylates downstream targets like vasodilator-stimulated phosphoprotein (VASP) and promotes smooth muscle relaxation. The synergy between H2S and nitric oxide (NO) signaling further amplifies vasodilation. H2S enhances NO bioavailability by inhibiting its degradation and stimulating endothelial nitric oxide synthase (eNOS) activity, increasing cGMP levels and potent vasodilatory responses. Protein sulfhydration, a post-translational modification, plays a crucial role in cell signaling. H2S S-sulfurates oxidized cysteine residues, while polysulfides (H2Sn) are responsible for S-sulfurating reduced cysteine residues. Sulfhydration of key proteins like K_ATP channels and sGC enhances their activity, contributing to the overall vasodilatory effect. Furthermore, H2S interaction with endothelium-derived hyperpolarizing factor (EDHF) pathways adds another layer to its vasodilatory mechanism. By enhancing EDHF activity, H2S facilitates the hyperpolarization and relaxation of VSMCs through gap junctions between endothelial cells and VSMCs. Recent findings suggest that H2S can also modulate transient receptor potential (TRP) channels, particularly TRPV4 channels, in endothelial cells. Activating these channels by H2S promotes calcium entry, stimulating the production of vasodilatory agents like NO and prostacyclin, thereby regulating vascular tone. The comprehensive understanding of H2S-induced vasodilation mechanisms highlights its therapeutic potential. The multifaceted approach of H2S in modulating vascular tone presents a promising strategy for developing novel treatments for hypertension, ischemic conditions, and other vascular disorders. The interaction of H2S with ion channels, cyclic nucleotide signaling, NO pathways, ROS (Reactive Oxygen Species) scavenging, protein sulfhydration, and EDHF underscores its complexity and therapeutic relevance. In conclusion, the intricate signaling paradigms of H2S-induced vasodilation offer valuable insights into its physiological role and therapeutic potential, promising innovative approaches for managing various vascular diseases through the modulation of vascular tone.
Keywords: ROS scavenging; endothelial function; hydrogen sulfide (H2S); protein sulfhydration; vasodilation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

