Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer's Disease
- PMID: 39456417
- PMCID: PMC11504317
- DOI: 10.3390/antiox13101164
Withania somnifera (Ashwagandha) Improves Spatial Memory, Anxiety and Depressive-like Behavior in the 5xFAD Mouse Model of Alzheimer's Disease
Abstract
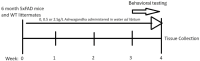
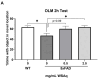
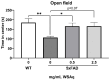
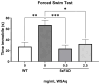
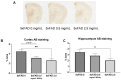
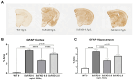
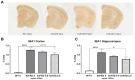
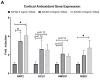
Withania somnifera (WS), also known as ashwagandha, is a popular botanical supplement used to treat various conditions including memory loss, anxiety and depression. Previous studies from our group showed an aqueous extract of WS root (WSAq) enhances cognition and alleviates markers for depression in Drosophila. Here, we sought to confirm these effects in the 5xFAD mouse model of β-amyloid (Aβ) accumulation. Six- to seven-month-old male and female 5xFAD mice were treated with WSAq in their drinking water at 0 mg/mL, 0.5 mg/mL or 2.5 mg/mL for four weeks. In the fourth week of treatment, spatial memory, anxiety and depressive-like symptoms were evaluated. At the conclusion of behavioral testing, brain tissue was harvested, immunohistochemistry was performed, and the cortical expression of antioxidant response genes was evaluated. Both concentrations of WSAq improved spatial memory and reduced depressive and anxiety-related behavior. These improvements were accompanied by a reduction in Aβ plaque burden in the hippocampus and cortex and an attenuation of activation of microglia and astrocytes. Antioxidant response genes were upregulated in the cortex of WSAq-treated mice. Oral WSAq treatment could be beneficial as a therapeutic option in AD for improving disease pathology and behavioral symptoms. Future studies focused on dose optimization of WSAq administration and further assessment of the mechanisms by which WSAq elicits its beneficial effects will help inform the clinical potential of this promising botanical therapy.
Keywords: 5xFAD mice; Alzheimer’s disease; anxiety; ashwagandha; beta-amyloid; depression; memory; neuroinflammation; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Prolonged Treatment with Centella asiatica Improves Memory, Reduces Amyloid-β Pathology, and Activates NRF2-Regulated Antioxidant Response Pathway in 5xFAD Mice.J Alzheimers Dis. 2021;81(4):1453-1468. doi: 10.3233/JAD-210271. J Alzheimers Dis. 2021. PMID: 33935097 Free PMC article.
-
Centella asiatica attenuates hippocampal mitochondrial dysfunction and improves memory and executive function in β-amyloid overexpressing mice.Mol Cell Neurosci. 2018 Dec;93:1-9. doi: 10.1016/j.mcn.2018.09.002. Epub 2018 Sep 22. Mol Cell Neurosci. 2018. PMID: 30253196 Free PMC article.
-
Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats.J Complement Integr Med. 2015 Jun;12(2):117-25. doi: 10.1515/jcim-2014-0075. J Complement Integr Med. 2015. PMID: 25803089
-
Withania somnifera Extracts Promote Resilience against Age-Related and Stress-Induced Behavioral Phenotypes in Drosophila melanogaster; a Possible Role of Other Compounds besides Withanolides.Nutrients. 2022 Sep 22;14(19):3923. doi: 10.3390/nu14193923. Nutrients. 2022. PMID: 36235577 Free PMC article.
-
Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia.Curr Neuropharmacol. 2021;19(9):1468-1495. doi: 10.2174/1570159X19666210712151556. Curr Neuropharmacol. 2021. PMID: 34254920 Free PMC article. Review.
Cited by
-
Quantifying Withanolides in Plasma: Pharmacokinetic Studies and Analytical Methods.Nutrients. 2024 Nov 8;16(22):3836. doi: 10.3390/nu16223836. Nutrients. 2024. PMID: 39599622 Free PMC article. Review.
-
Preclinical Evidence of Withania somnifera and Cordyceps spp.: Neuroprotective Properties for the Management of Alzheimer's Disease.Int J Mol Sci. 2025 Jun 4;26(11):5403. doi: 10.3390/ijms26115403. Int J Mol Sci. 2025. PMID: 40508211 Free PMC article. Review.
-
N-Acetylcysteine Attenuates Aβ-Mediated Oxidative Stress, Blood-Brain Barrier Leakage, and Renal Dysfunction in 5xFAD Mice.Int J Mol Sci. 2025 May 3;26(9):4352. doi: 10.3390/ijms26094352. Int J Mol Sci. 2025. PMID: 40362589 Free PMC article.
-
A Novel Ashwagandha (Withania somnifera) Formulation Mitigates Sleep Deprivation-Induced Cognitive Impairment and Oxidative Stress in a Rat Model.Biomolecules. 2025 May 12;15(5):710. doi: 10.3390/biom15050710. Biomolecules. 2025. PMID: 40427603 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

