Modulatory Impact of Oxidative Stress on Action Potentials in Pathophysiological States: A Comprehensive Review
- PMID: 39456426
- PMCID: PMC11504047
- DOI: 10.3390/antiox13101172
Modulatory Impact of Oxidative Stress on Action Potentials in Pathophysiological States: A Comprehensive Review
Abstract
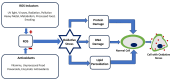
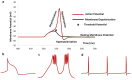
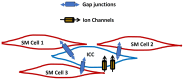
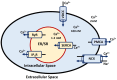
Oxidative stress, characterized by an imbalance between the production of reactive oxygen species (ROS) and the body's antioxidant defenses, significantly affects cellular function and viability. It plays a pivotal role in modulating membrane potentials, particularly action potentials (APs), essential for properly functioning excitable cells such as neurons, smooth muscles, pancreatic beta cells, and myocytes. The interaction between oxidative stress and AP dynamics is crucial for understanding the pathophysiology of various conditions, including neurodegenerative diseases, cardiac arrhythmias, and ischemia-reperfusion injuries. This review explores how oxidative stress influences APs, focusing on alterations in ion channel biophysics, gap junction, calcium dynamics, mitochondria, and Interstitial Cells of Cajal functions. By integrating current research, we aim to elucidate how oxidative stress contributes to disease progression and discuss potential therapeutic interventions targeting this interaction.
Keywords: APs; biophysics; ion channel; oxidative stress; pathophysiology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases.Int J Med Sci. 2019 Sep 20;16(10):1386-1396. doi: 10.7150/ijms.36516. eCollection 2019. Int J Med Sci. 2019. PMID: 31692944 Free PMC article. Review.
-
Effect of oxidative stress on male reproduction.World J Mens Health. 2014 Apr;32(1):1-17. doi: 10.5534/wjmh.2014.32.1.1. Epub 2014 Apr 25. World J Mens Health. 2014. PMID: 24872947 Free PMC article. Review.
-
Oxidative stress in the mitochondrial matrix underlies ischemia/reperfusion-induced mitochondrial instability.J Biol Chem. 2023 Jan;299(1):102780. doi: 10.1016/j.jbc.2022.102780. Epub 2022 Dec 7. J Biol Chem. 2023. PMID: 36496071 Free PMC article.
-
Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders.Neurosci Biobehav Rev. 2022 Nov;142:104892. doi: 10.1016/j.neubiorev.2022.104892. Epub 2022 Sep 28. Neurosci Biobehav Rev. 2022. PMID: 36181925 Review.
-
Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
Cited by
-
Congestive Heart Failure and Arrhythmias Among Hospitalized Patients With Carcinoid Syndrome.Cureus. 2025 Apr 9;17(4):e81958. doi: 10.7759/cureus.81958. eCollection 2025 Apr. Cureus. 2025. PMID: 40206499 Free PMC article.
-
Review of electrophysiological models to study membrane potential changes in breast cancer cell transformation and tumor progression.Front Physiol. 2025 Mar 5;16:1536165. doi: 10.3389/fphys.2025.1536165. eCollection 2025. Front Physiol. 2025. PMID: 40110186 Free PMC article. Review.
-
Modulation of Redox-Sensitive Cardiac Ion Channels.Antioxidants (Basel). 2025 Jul 8;14(7):836. doi: 10.3390/antiox14070836. Antioxidants (Basel). 2025. PMID: 40722941 Free PMC article. Review.
-
Nanocarbon-Driven Recovery of Mechano-Kinetic Properties of Injured Rat Gastrocnemius Muscle.Int J Mol Sci. 2025 Jun 9;26(12):5511. doi: 10.3390/ijms26125511. Int J Mol Sci. 2025. PMID: 40564975 Free PMC article.
-
Relationship Between Oxidative Stress and Severity of Diabetic Foot Ulcers Among Patients With Type-2 Diabetes Mellitus in Japan: A Cross-Sectional Study.Health Sci Rep. 2025 Jul 2;8(7):e70935. doi: 10.1002/hsr2.70935. eCollection 2025 Jul. Health Sci Rep. 2025. PMID: 40606312 Free PMC article.
References
-
- Salmaninejad A., Ilkhani K., Marzban H., Navashenaq J.G., Rahimirad S., Radnia F., Yousefi M., Bahmanpour Z., Azhdari S., Sahebkar A. Genomic instability in cancer: Molecular mechanisms and therapeutic potentials. Curr. Pharm. Des. 2021;27:3161–3169. doi: 10.2174/1381612827666210426100206. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

