NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia-Complementing Prostacyclin
- PMID: 39456432
- PMCID: PMC11504732
- DOI: 10.3390/antiox13101178
NADPH Oxidase 4: Crucial for Endothelial Function under Hypoxia-Complementing Prostacyclin
Abstract
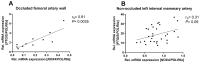
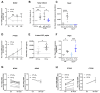
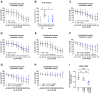
Aim: The primary endothelial NADPH oxidase isoform 4 (NOX4) is notably induced during hypoxia, with emerging evidence suggesting its vasoprotective role through H2O2 production. Therefore, we aimed to elucidate NOX4's significance in endothelial function under hypoxia. Methods: Human vessels, in addition to murine vessels from Nox4-/- mice, were explored. On a functional level, Mulvany myograph experiments were performed. To obtain mechanistical insights, human endothelial cells were cultured under hypoxia with inhibitors of hypoxia-inducible factors. Additionally, endothelial cells were cultured under combined hypoxia and laminar shear stress conditions. Results: In human occluded vessels, NOX4 expression strongly correlated with prostaglandin I2 synthase (PTGIS). Hypoxia significantly elevated NOX4 and PTGIS expression and activity in human endothelial cells. Inhibition of prolyl hydroxylase domain (PHD) enzymes, which stabilize hypoxia-inducible factors (HIFs), increased NOX4 and PTGIS expression even under normoxic conditions. NOX4 mRNA expression was reduced by HIF1a inhibition, while PTGIS mRNA expression was only affected by the inhibition of HIF2a under hypoxia. Endothelial function assessments revealed hypoxia-induced endothelial dysfunction in mesenteric arteries from wild-type mice. Mesenteric arteries from Nox4-/- mice exhibited an altered endothelial function under hypoxia, most prominent in the presence of cyclooxygenase inhibitor diclofenac to exclude the impact of prostacyclin. Restored protective laminar shear stress, as it might occur after thrombolysis, angioplasty, or stenting, attenuated the hypoxic response in endothelial cells, reducing HIF1a expression and its target NOX4 while enhancing eNOS expression. Conclusions: Hypoxia strongly induces NOX4 and PTGIS, with a close correlation between both factors in occluded, hypoxic human vessels. This relationship ensured endothelium-dependent vasodilation under hypoxic conditions. Protective laminar blood flow restores eNOS expression and mitigates the hypoxic response on NOX4 and PTGIS.
Keywords: NADPH oxidase 4; PTGIS; endothelial function; human endothelial cells; hypoxia; laminar flow.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The Vascular Function of Resistance Arteries Depends on NADPH Oxidase 4 and Is Exacerbated by Perivascular Adipose Tissue.Antioxidants (Basel). 2024 Apr 23;13(5):503. doi: 10.3390/antiox13050503. Antioxidants (Basel). 2024. PMID: 38790608 Free PMC article.
-
[Relationship between expression of endothelial nitric oxide synthase and NADPH oxidase in lungs of mice exposed to chronic hypoxia].Zhongguo Dang Dai Er Ke Za Zhi. 2015 Sep;17(9):1001-6. Zhongguo Dang Dai Er Ke Za Zhi. 2015. PMID: 26412187 Chinese.
-
NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice.Eur Heart J. 2016 Jun 7;37(22):1753-61. doi: 10.1093/eurheartj/ehv564. Epub 2015 Nov 17. Eur Heart J. 2016. PMID: 26578199 Free PMC article.
-
Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4.Diabetes. 2010 Jun;59(6):1528-38. doi: 10.2337/db09-1057. Epub 2010 Mar 23. Diabetes. 2010. PMID: 20332345 Free PMC article.
-
NADPH oxidase 4 mediates the protective effects of physical activity against obesity-induced vascular dysfunction.Cardiovasc Res. 2020 Aug 1;116(10):1767-1778. doi: 10.1093/cvr/cvz322. Cardiovasc Res. 2020. PMID: 31800011
References
Grants and funding
LinkOut - more resources
Full Text Sources

