Minimizing Oxidative Stress in the Lens: Alternative Measures for Elevating Glutathione in the Lens to Protect against Cataract
- PMID: 39456447
- PMCID: PMC11505578
- DOI: 10.3390/antiox13101193
Minimizing Oxidative Stress in the Lens: Alternative Measures for Elevating Glutathione in the Lens to Protect against Cataract
Abstract
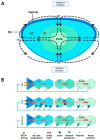
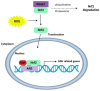
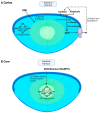
Oxidative stress plays a major role in the formation of the cataract that is the result of advancing age, diabetes or which follows vitrectomy surgery. Glutathione (GSH) is the principal antioxidant in the lens, and so supplementation with GSH would seem like an intuitive strategy to counteract oxidative stress there. However, the delivery of glutathione to the lens is fraught with difficulties, including the limited bioavailability of GSH caused by its rapid degradation, anatomical barriers of the anterior eye that result in insufficient delivery of GSH to the lens, and intracellular barriers within the lens that limit delivery of GSH to its different regions. Hence, more attention should be focused on alternative methods by which to enhance GSH levels in the lens. In this review, we focus on the following three strategies, which utilize the natural molecular machinery of the lens to enhance GSH and/or antioxidant potential in its different regions: the NRF2 pathway, which regulates the transcription of genes involved in GSH homeostasis; the use of lipid permeable cysteine-based analogues to increase the availability of cysteine for GSH synthesis; and the upregulation of the lens's internal microcirculation system, which is a circulating current of Na+ ions that drives water transport in the lens and with it the potential delivery of cysteine or GSH. The first two strategies have the potential to restore GSH levels in the epithelium and cortex, while the ability to harness the lens's internal microcirculation system offers the exciting potential to deliver and elevate antioxidant levels in its nucleus. This is an important distinction, as the damage phenotypes for age-related (nuclear) and diabetic (cortical) cataract indicate that antioxidant delivery must be targeted to different regions of the lens in order to alleviate oxidative stress. Given our increasing aging and diabetic populations it has become increasingly important to consider how the natural machinery of the lens can be utilized to restore GSH levels in its different regions and to afford protection from cataract.
Keywords: Nrf2; cataracts; cystine/cysteine; glutathione; lens microcirculation system.
Conflict of interest statement
J.C.L. and P.J.D. have collaborated with Nacuity Pharmaceuticals, Inc. in the testing of NACA and diNACA analogues on the lens.
Figures

References
-
- Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

