Synergistic Dual Targeting of Thioredoxin and Glutathione Systems Irrespective of p53 in Glioblastoma Stem Cells
- PMID: 39456455
- PMCID: PMC11504866
- DOI: 10.3390/antiox13101201
Synergistic Dual Targeting of Thioredoxin and Glutathione Systems Irrespective of p53 in Glioblastoma Stem Cells
Abstract
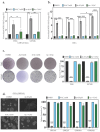
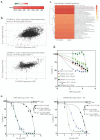
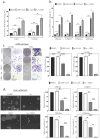
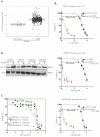
Glioblastoma (GBM) is an incurable primary brain cancer characterized by increased reactive oxygen species (ROS) production. The redox-sensitive tumor suppressor gene TP53, wild-type (wt) for 70% of patients, regulates redox homeostasis. Glioblastoma stem cells (GSCs) increase thioredoxin (Trx) and glutathione (GSH) antioxidant systems as survival redox-adaptive mechanisms to maintain ROS below the cytotoxic threshold. Auranofin, an FDA-approved anti-rheumatoid drug, inhibits thioredoxin reductase 1 (TrxR1). L-buthionine sulfoximine (L-BSO) and the natural product piperlongumine (PPL) inhibit the GSH system. We evaluated the cytotoxic effects of Auranofin alone and in combination with L-BSO or PPL in GBM cell lines and GSCs with a known TP53 status. The Cancer Genome Atlas/GBM analysis revealed a significant positive correlation between wtp53 and TrxR1 expression in GBM. Auranofin induced ROS-dependent cytotoxicity within a micromolar range in GSCs. Auranofin decreased TrxR1 expression, AKT (Ser-473) phosphorylation, and increased p53, p21, and PARP-1 apoptotic cleavage in wtp53-GSCs, while mutant-p53 was decreased in a mutant-p53 GSC line. Additionally, p53-knockdown in a wtp53-GSC line decreased TrxR1 expression and significantly increased sensitivity to Auranofin, suggesting the role of wtp53 as a negative redox-sensitive mechanism in response to Auranofin in GSCs. The combination of Auranofin and L-BSO synergistically increased ROS, decreased IC50s, and induced long-term cytotoxicity irrespective of p53 in GBM cell lines and GSCs. Intriguingly, Auranofin increased the expression of glutathione S-transferase pi-1 (GSTP-1), a target of PPL. Combining Auranofin with PPL synergistically decreased IC50s to a nanomolar range in GSCs, supporting the potential to repurpose Auranofin and PPL in GBM.
Keywords: Glioblastoma; Glioblastoma stem cells (GSCs); antioxidant; auranofin; drug repurposing; glutathione (GSH); oxidative stress; piperlongumine; reactive oxygen species (ROS); thioredoxin reductase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Alves A.L.V., Gomes I.N.F., Carloni A.C., Rosa M.N., da Silva L.S., Evangelista A.F., Reis R.M., Silva V.A.O. Role of Glioblastoma Stem Cells in Cancer Therapeutic Resistance: A Perspective on Antineoplastic Agents from Natural Sources and Chemical Derivatives. Stem Cell Res. Ther. 2021;12:206. doi: 10.1186/s13287-021-02231-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

