The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology
- PMID: 39456461
- PMCID: PMC11505517
- DOI: 10.3390/antiox13101208
The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology
Abstract
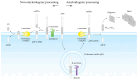
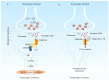
Alzheimer's disease (AD) is a progressive neurodegenerative disease, and it is currently the seventh leading cause of death worldwide. It is characterized by the extracellular aggregation of the amyloid β-peptide (Aβ) into oligomers and fibrils that cause synaptotoxicity and neuronal death. Aβ exhibits a dual role in promoting oxidative stress and inflammation. This review aims to unravel the intricate connection between these processes and their contribution to AD progression. The review delves into oxidative stress in AD, focusing on the involvement of metals, mitochondrial dysfunction, and biomolecule oxidation. The distinct yet overlapping concept of nitro-oxidative stress is also discussed, detailing the roles of nitric oxide, mitochondrial perturbations, and their cumulative impact on Aβ production and neurotoxicity. Inflammation is examined through astroglia and microglia function, elucidating their response to Aβ and their contribution to oxidative stress within the AD brain. The blood-brain barrier and oligodendrocytes are also considered in the context of AD pathophysiology. We also review current diagnostic methodologies and emerging therapeutic strategies aimed at mitigating oxidative stress and inflammation, thereby offering potential treatments for halting or slowing AD progression. This comprehensive synthesis underscores the pivotal role of Aβ in bridging oxidative stress and inflammation, advancing our understanding of AD and informing future research and treatment paradigms.
Keywords: Alzheimer’s disease; BACE1; amyloid β-peptide; neurodegeneration; nitro-oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- World Health Organization . Global Status Report on the Public Health Response to Dementia. World Health Organization; Geneva, Switzerland: 2021.
-
- Long S., Benoist C., Weidner W. World Alzheimer Report 2023 Reducing Dementia Risk: Never Too Early, Never Too Late. Alzheimer’s Disease International; London, UK: 2023.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

