Frequency-Dependent Antioxidant Responses in HT-1080 Human Fibrosarcoma Cells Exposed to Weak Radio Frequency Fields
- PMID: 39456490
- PMCID: PMC11504554
- DOI: 10.3390/antiox13101237
Frequency-Dependent Antioxidant Responses in HT-1080 Human Fibrosarcoma Cells Exposed to Weak Radio Frequency Fields
Abstract
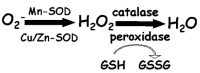
This study explores the complex relationship between radio frequency (RF) exposure and cancer cells, focusing on the HT-1080 human fibrosarcoma cell line. We investigated the modulation of reactive oxygen species (ROS) and key antioxidant enzymes, including superoxide dismutase (SOD), peroxidase, and glutathione (GSH), as well as mitochondrial superoxide levels and cell viability. Exposure to RF fields in the 2-5 MHz range at very weak intensities (20 nT) over 4 days resulted in distinct, frequency-specific cellular effects. Significant increases in SOD and GSH levels were observed at 4 and 4.5 MHz, accompanied by reduced mitochondrial superoxide levels and enhanced cell viability, suggesting improved mitochondrial function. In contrast, lower frequencies like 2.5 MHz induced oxidative stress, evidenced by GSH depletion and increased mitochondrial superoxide levels. The findings demonstrate that cancer cells exhibit frequency-specific sensitivity to RF fields even at intensities significantly below current safety standards, highlighting the need to reassess exposure limits. Additionally, our analysis of the radical pair mechanism (RPM) offers deeper insight into RF-induced cellular responses. The modulation of ROS and antioxidant enzyme activities is significant for cancer treatment and has broader implications for age-related diseases, where oxidative stress is a central factor in cellular degeneration. The findings propose that RF fields may serve as a therapeutic tool to selectively modulate oxidative stress and mitochondrial function in cancer cells, with antioxidants playing a key role in mitigating potential adverse effects.
Keywords: antioxidant enzymes; cancer cells; mitochondrial superoxide; oxidative stress; peroxidase; radical pair mechanism; radio frequency fields; reactive oxygen species; reduced glutathione; superoxide dismutase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Romanenko S., Begley R., Harvey A.R., Hool L., Wallace V.P. The Interaction between Electromagnetic Fields at Megahertz, Gigahertz and Terahertz Frequencies with Cells, Tissues and Organisms: Risks and Potential. J. R. Soc. Interface. 2017;14:20170585. doi: 10.1098/rsif.2017.0585. - DOI - PMC - PubMed
-
- Lambert N., Chen Y.-N., Cheng Y.-C., Li C.-M., Chen G.-Y., Nori F. Quantum Biology. Nat. Phys. 2013;9:10–18. doi: 10.1038/nphys2474. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

