Pristine Photopolymerizable Gelatin Hydrogels: A Low-Cost and Easily Modifiable Platform for Biomedical Applications
- PMID: 39456491
- PMCID: PMC11505247
- DOI: 10.3390/antiox13101238
Pristine Photopolymerizable Gelatin Hydrogels: A Low-Cost and Easily Modifiable Platform for Biomedical Applications
Abstract
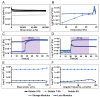
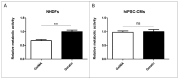
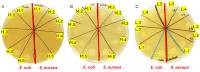
The study addresses the challenge of temperature sensitivity in pristine gelatin hydrogels, widely used in biomedical applications due to their biocompatibility, low cost, and cell adhesion properties. Traditional gelatin hydrogels dissolve at physiological temperatures, limiting their utility. Here, we introduce a novel method for creating stable hydrogels at 37 °C using pristine gelatin through photopolymerization without requiring chemical modifications. This approach enhances consistency and simplifies production and functionalization of the gelatin with bioactive molecules. The stabilization mechanism involves the partial retention of the triple-helix structure of gelatin below 25 °C, which provides specific crosslinking sites. Upon activation by visible light, ruthenium (Ru) acts as a photosensitizer that generates sulphate radicals from sodium persulphate (SPS), inducing covalent bonding between tyrosine residues and "locking" the triple-helix conformation. The primary focus of this work is the characterization of the mechanical properties, swelling ratio, and biocompatibility of the photopolymerized gelatin hydrogels. Notably, these hydrogels supported better cell viability and elongation in normal human dermal fibroblasts (NHDFs) compared to GelMA, and similar performance was observed for human pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). As a proof of concept for functionalization, gelatin was modified with selenous acid (GelSe), which demonstrated antioxidant and antimicrobial capacities, particularly against E. coli and S. aureus. These results suggest that pristine gelatin hydrogels, enhanced through this new photopolymerization method and functionalized with bioactive molecules, hold potential for advancing regenerative medicine and tissue engineering by providing robust, biocompatible scaffolds for cell culture and therapeutic applications.
Keywords: biomaterial; cardiovascular; chronic wounds; gelatin; hydrogel; selenium; tissue engineering.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

