Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis
- PMID: 39456494
- PMCID: PMC11504967
- DOI: 10.3390/antiox13101241
Hydrogen Sulfide Modulates Astrocytic Toxicity in Mouse Spinal Cord Cultures: Implications for Amyotrophic Lateral Sclerosis
Abstract
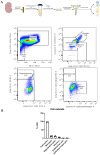
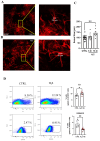
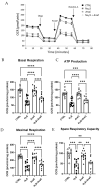
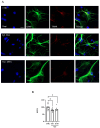
Hydrogen sulfide (H2S), a known inhibitor of the electron transport chain, is endogenously produced in the periphery as well as in the central nervous system, where is mainly generated by glial cells. It affects, as a cellular signaling molecule, many different biochemical processes. In the central nervous system, depending on its concentration, it can be protective or damaging to neurons. In the study, we have demonstrated, in a primary mouse spinal cord cultures, that it is particularly harmful to motor neurons, is produced by glial cells, and is stimulated by inflammation. However, its role on glial cells, especially astrocytes, is still under-investigated. The present study was designed to evaluate the impact of H2S on astrocytes and their phenotypic heterogeneity, together with the functionality and homeostasis of mitochondria in primary spinal cord cultures. We found that H2S modulates astrocytes' morphological changes and their phenotypic transformation, exerts toxic properties by decreasing ATP production and the mitochondrial respiration rate, disturbs mitochondrial depolarization, and alters the energetic metabolism. These results further support the hypothesis that H2S is a toxic mediator, mainly released by astrocytes, possibly acting as an autocrine factor toward astrocytes, and probably involved in the non-cell autonomous mechanisms leading to motor neuron death.
Keywords: astrocytes; hydrogen sulfide; mitochondria; motor neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bełtowski J. Vascular Effects of Hydrogen Sulfide. Humana; New York, NY, USA: 2019. Synthesis, Metabolism, and Signaling Mechanisms of Hydrogen Sulfide: An Overview; pp. 1–8. - PubMed
LinkOut - more resources
Full Text Sources

