Aged Gut Microbiome Induces Metabolic Impairment and Hallmarks of Vascular and Intestinal Aging in Young Mice
- PMID: 39456503
- PMCID: PMC11505429
- DOI: 10.3390/antiox13101250
Aged Gut Microbiome Induces Metabolic Impairment and Hallmarks of Vascular and Intestinal Aging in Young Mice
Abstract
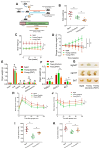
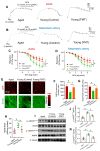
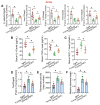
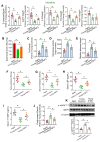
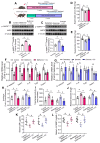
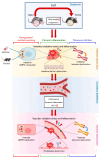
Aging, an independent risk factor for cardiometabolic diseases, refers to a progressive deterioration in physiological function, characterized by 12 established hallmarks. Vascular aging is driven by endothelial dysfunction, telomere dysfunction, oxidative stress, and vascular inflammation. This study investigated whether aged gut microbiome promotes vascular aging and metabolic impairment. Fecal microbiome transfer (FMT) was conducted from aged (>75 weeks old) to young C57BL/6 mice (8 weeks old) for 6 weeks. Wire myography was used to evaluate endothelial function in aortas and mesenteric arteries. ROS levels were measured by dihydroethidium (DHE) staining and lucigenin-enhanced chemiluminescence. Vascular and intestinal telomere function, in terms of relative telomere length, telomerase reverse transcriptase expression and telomerase activity, were measured. Systemic inflammation, endotoxemia and intestinal integrity of mice were assessed. Gut microbiome profiles were studied by 16S rRNA sequencing. Some middle-aged mice (40-42 weeks old) were subjected to chronic metformin treatment and exercise training for 4 weeks to evaluate their anti-aging benefits. Six-week FMT impaired glucose homeostasis and caused vascular dysfunction in aortas and mesenteric arteries in young mice. FMT triggered vascular inflammation and oxidative stress, along with declined telomerase activity and shorter telomere length in aortas. Additionally, FMT impaired intestinal integrity, and triggered AMPK inactivation and telomere dysfunction in intestines, potentially attributed to the altered gut microbial profiles. Metformin treatment and moderate exercise improved integrity, AMPK activation and telomere function in mouse intestines. Our data highlight aged microbiome as a mechanism that accelerates intestinal and vascular aging, suggesting the gut-vascular connection as a potential intervention target against cardiovascular aging and complications.
Keywords: AMPK; aging; dysbiosis; endothelial cell; microbiome; telomeres.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

