Exploring the 3,5-Dibromo-4,6-dimethoxychalcones and Their Flavone Derivatives as Dual α-Glucosidase and α-Amylase Inhibitors with Antioxidant and Anticancer Potential
- PMID: 39456508
- PMCID: PMC11505200
- DOI: 10.3390/antiox13101255
Exploring the 3,5-Dibromo-4,6-dimethoxychalcones and Their Flavone Derivatives as Dual α-Glucosidase and α-Amylase Inhibitors with Antioxidant and Anticancer Potential
Abstract
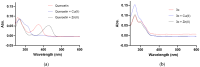
The rising levels of type 2 diabetes mellitus (T2DM) and the poor medical effects of the commercially available antidiabetic drugs necessitate the development of potent analogs to treat this multifactorial metabolic disorder. It has been demonstrated that targeting two or more biochemical targets associated with the onset and progression of diabetes along with oxidative stress and/or cancer could be a significant strategy for treating complications related to this metabolic disorder. The 3,5-dibromo-4,6-dimethoxychalcones (2a-f) and the corresponding flavone derivatives (3a-f) were synthesized and characterized using spectroscopic (NMR, HR-MS and FT-IR) techniques. The inhibitory effect of both series of compounds against α-glucosidase and α-amylase was evaluated in vitro through enzymatic assays. Selected compounds were also evaluated for potential to activate or inhibit superoxide dismutase. Compound 3c was selected as a representative model for the flavone series and evaluated spectrophotometrically for potential to coordinate Cu(II) and/or Zn(II) ions implicated in the metal-catalyzed free radical generation. A plausible mechanism for metal-chelation of the test compounds is presented. Furthermore, the most active compounds from each series against the test carbohydrate-hydrolyzing enzymes were selected and evaluated for their antigrowth effect on the human breast (MCF-7) and lung (A549) cancer cell lines and for cytotoxicity against the African Green Monkey kidney (Vero) cell line. The parent chalcone 2a and flavone derivatives 3a, 3c and 3e exhibited relatively high inhibitory activity against the MCF-7 cells with IC50 values of 4.12 ± 0.55, 8.50 ± 0.82, 5.10 ± 0.61 and 6.96 ± 0.66 μM, respectively. The chalcones 2a and 2c exhibited significant cytotoxicity against the A549 cells with IC50 values of 7.40 ± 0.67 and 9.68 ± 0.80 μM, respectively. Only flavone 3c exhibited relatively strong and comparable cytotoxicity against the MCF-7 and A549 cell lines with IC50 values of 6.96 ± 0.66 and 6.42 ± 0.79 μM, respectively. Both series of compounds exhibited strong activity against the MCF-7 and A549 cell lines compared to the analogous quercetin (IC50 = 35.40 ± 1.78 and 35.38 ± 1.78 μM, respectively) though moderate compared to nintedanib (IC50 = 0.53 ± 0.11 and 0.74 ± 0.15 μM, respectively). The test compounds generally exhibited reduced cytotoxicity against the Vero cells compared to this anticancer drug. Molecular docking revealed strong alignment of the test compounds with the enzyme backbone to engage in hydrogen bonding interaction/s and hydrophobic contacts with the residues in the active sites of α-glucosidase and α-amylase. The test compounds possess favorable drug-likeness properties, supporting their potential as therapeutic candidates against T2DM.
Keywords: 3,5-dibromo-4,6-dimethoxychalcones; 6,8-dibromo-5,7-dimethoxyflavones; antioxidant activity; carbohydrate-hydrolyzing enzymes; cytotoxicity; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
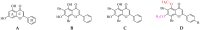




References
-
- Chaudhury A., Duvoor C., Dendi V.S.R., Kraleti S., Chada A., Ravilla R., Marco A., Shekhawat N.S., Montales M.T., Kuriakose K., et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus, management. Front. Endocrinol. 2017;8:6. doi: 10.3389/fendo.2017.00006. - DOI - PMC - PubMed
-
- Lam T.-P., Tran N.-V.N., Pham L.-H.D., Lai N.V.-T., Dang B.-T.N., Truong N.-L.N., Nguyen-Vo S.-K., Hoang T.-L., Mai T.T., Tran T.-D. Flavonoids as dual-target inhibitors against α-glucosidase and α-amylase: A systematic review of in vitro studies. Nat. Prod. Bioprospect. 2024;14:4. doi: 10.1007/s13659-023-00424-w. - DOI - PMC - PubMed
-
- Mai T.T., Phan M.-H., Thai T.T., Lam T.-P., Lai N.V.-T., Nguyen T.-T., Nguyen T.-V.-P., Vo C.-V.T., Thai K.-M., Tran T.-D. Discovery of novel flavonoid derivatives as potential dual inhibitors against α-glucosidase and α-amylase: Virtual screening, synthesis, and biological evaluation. Mol. Divers. 2024;28:1629–1650. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

