Association Between NOX2-Mediated Oxidative Stress, Low-Grade Endotoxemia, Hypoalbuminemia, and Clotting Activation in COVID-19
- PMID: 39456513
- PMCID: PMC11505442
- DOI: 10.3390/antiox13101260
Association Between NOX2-Mediated Oxidative Stress, Low-Grade Endotoxemia, Hypoalbuminemia, and Clotting Activation in COVID-19
Abstract
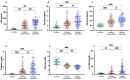
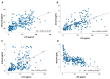
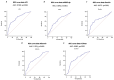
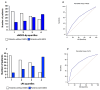
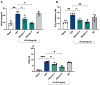
Low-grade endotoxemia by lipopolysaccharide (LPS) has been detected in COVID-19 and could favor thrombosis via eliciting a pro-inflammatory and pro-coagulant state. The aim of this study was to analyze the mechanism accounting for low-grade endotoxemia and its relationship with oxidative stress and clotting activation thrombosis in COVID-19. We measured serum levels of sNOX2-dp, zonulin, LPS, D-dimer, and albumin in 175 patients with COVID-19, classified as having or not acute respiratory distress syndrome (ARDS), and 50 healthy subjects. Baseline levels of sNOX2-dp, LPS, zonulin, D-dimer, albumin, and hs-CRP were significantly higher in COVID-19 compared to controls. In COVID-19 patients with ARDS, sNOX2-dp, LPS, zonulin, D-dimer, and hs-CRP were significantly higher compared to COVID-19 patients without ARDS. Conversely, concentration of albumin was lower in patients with ARDS compared with those without ARDS and inversely associated with LPS. In the COVID-19 cohort, the number of patients with ARDS progressively increased according to sNOX2-dp and LPS quartiles; a significant correlation between LPS and sNOX2-dp and LPS and D-dimer was detected in COVID-19. In a multivariable logistic regression model, LPS/albumin levels and D-dimer predicted thrombotic events. In COVID-19 patients, LPS is significantly associated with a hypercoagulation state and disease severity. In vitro, LPS can increase endothelial oxidative stress and coagulation biomarkers that were reduced by the treatment with albumin. In conclusion, impaired gut barrier permeability, increased NOX2 activation, and low serum albumin may account for low-grade endotoxemia and may be implicated in thrombotic events in COVID-19.
Keywords: D-dimer; NOX2 activation; albumin; gut permeability; low-grade endotoxemia; oxidative stress; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. - DOI - PMC - PubMed
-
- Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. - DOI - PMC - PubMed
-
- Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Bertuzzi A., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

