Rosmarinic Acid Attenuates Salmonella enteritidis-Induced Inflammation via Regulating TLR9/NF-κB Signaling Pathway and Intestinal Microbiota
- PMID: 39456517
- PMCID: PMC11504439
- DOI: 10.3390/antiox13101265
Rosmarinic Acid Attenuates Salmonella enteritidis-Induced Inflammation via Regulating TLR9/NF-κB Signaling Pathway and Intestinal Microbiota
Abstract
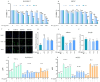
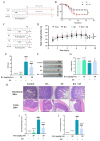
Salmonella enteritidis (SE) infection disrupts the homeostasis of the intestinal microbiota, causing an intestinal inflammatory response and posing a great threat to human and animal health. The unreasonable use of antibiotics has led to an increase in the prevalence of drug-resistant SE, increasing the difficulty of controlling SE. Therefore, new drug strategies and research are urgently needed to control SE. Rosmarinic acid (RA) is a natural phenolic acid with various pharmacological activities, including antioxidant, anti-inflammatory and antibacterial properties. However, the protective effects and mechanism of RA on intestinal inflammation and the gut microbial disorders caused by SE have not been fully elucidated. In this study, RAW264.7 cells, MCECs and BALB/c mice were challenged with SE to assess the protective effects and mechanisms of RA. The results showed that RA enhanced the phagocytic ability of RAW264.7 cells, reduced the invasion and adhesion ability of SE in MCECs, and inhibited SE-induced inflammation in cells. Moreover, RA inhibited the activation of the NF-κB signaling pathway by upregulating TLR9 expression. Importantly, we found that RA provided protection against SE and increased the diversity and abundance of the intestinal microbiota in mice. Compared with infection control, RA significantly increased the abundance of Firmicutes and Acidibacteria and decreased the abundance of Proteobacteria, Epsilonbacteraeota and Bacteroidota. However, RA failed to alleviate SE-induced inflammation and lost its regulatory effects on the TLR9/NF-κB signaling pathway after destroying the gut microbiota with broad-spectrum antibiotics. These results indicated that RA attenuated SE-induced inflammation by regulating the TLR9/NF-κB signaling pathway and maintaining the homeostasis of the gut microbiota. Our study provides a new strategy for preventing SE-induced intestinal inflammation.
Keywords: Gut microbiota; NF-κB; Rosmarinic acid; Salmonella enteritidis; TLR9.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources

