Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy
- PMID: 39456564
- PMCID: PMC11506508
- DOI: 10.3390/cancers16203470
Synthetic and Natural Inhibitors of Mortalin for Cancer Therapy
Abstract
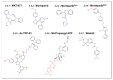
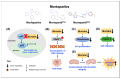
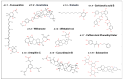
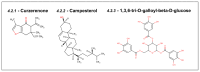
Upregulation of stress chaperone Mortalin has been closely linked to the malignant transformation of cells, tumorigenesis, the progression of tumors to highly aggressive stages, metastasis, drug resistance, and relapse. Various in vitro and in vivo assays have provided evidence of the critical role of Mortalin upregulation in promoting cancer cell characteristics, including proliferation, migration, invasion, and the inhibition of apoptosis, a consistent feature of most cancers. Given its critical role in several steps in oncogenesis and multi-modes of action, Mortalin presents a promising target for cancer therapy. Consequently, Mortalin inhibitors are emerging as potential anti-cancer drugs. In this review, we discuss various inhibitors of Mortalin (peptides, small RNAs, natural and synthetic compounds, and antibodies), elucidating their anti-cancer potentials.
Keywords: Hsp70; cancer; chaperone; mortalin; natural and synthetic inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Mortaparib, a novel dual inhibitor of mortalin and PARP1, is a potential drug candidate for ovarian and cervical cancers.J Exp Clin Cancer Res. 2019 Dec 19;38(1):499. doi: 10.1186/s13046-019-1500-9. J Exp Clin Cancer Res. 2019. PMID: 31856867 Free PMC article.
-
SMR peptide antagonizes mortalin promoted release of extracellular vesicles and affects mortalin protection from complement-dependent cytotoxicity in breast cancer cells and leukemia cells.Oncotarget. 2019 Sep 10;10(52):5419-5438. doi: 10.18632/oncotarget.27138. eCollection 2019 Sep 10. Oncotarget. 2019. PMID: 31534628 Free PMC article.
-
Stress chaperone mortalin contributes to epithelial-mesenchymal transition and cancer metastasis.Cancer Res. 2016 May;76(9):2754-2765. doi: 10.1158/0008-5472.CAN-15-2704. Epub 2016 Mar 9. Cancer Res. 2016. PMID: 26960973
-
Why is Mortalin a Potential Therapeutic Target for Cancer?Front Cell Dev Biol. 2022 Jun 29;10:914540. doi: 10.3389/fcell.2022.914540. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35859897 Free PMC article. Review.
-
The versatile stress protein mortalin as a chaperone therapeutic agent.Protein Pept Lett. 2009;16(5):517-29. doi: 10.2174/092986609788167770. Protein Pept Lett. 2009. PMID: 19442231 Review.
Cited by
-
The Role of p66Shc in Cancer: Molecular Mechanisms and Therapeutic Implications.J Cell Mol Med. 2025 Jul;29(14):e70737. doi: 10.1111/jcmm.70737. J Cell Mol Med. 2025. PMID: 40690563 Free PMC article. Review.
-
Blockade of the STAT3/BCL-xL Axis Leads to the Cytotoxic and Cisplatin-Sensitizing Effects of Fucoxanthin, a Marine-Derived Carotenoid, on Human Bladder Urothelial Carcinoma Cells.Mar Drugs. 2025 Jan 22;23(2):54. doi: 10.3390/md23020054. Mar Drugs. 2025. PMID: 39997178 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

