Maize Endophytic Plant Growth-Promoting Bacteria Peribacillus simplex Can Alleviate Plant Saline and Alkaline Stress
- PMID: 39456656
- PMCID: PMC11508032
- DOI: 10.3390/ijms252010870
Maize Endophytic Plant Growth-Promoting Bacteria Peribacillus simplex Can Alleviate Plant Saline and Alkaline Stress
Abstract
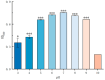
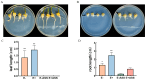
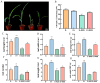
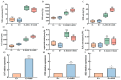
Soil salinization is currently one of the main abiotic stresses that restrict plant growth. Plant endophytic bacteria can alleviate abiotic stress. The aim of the current study was to isolate, characterize, and assess the plant growth-promoting and saline and alkaline stress-alleviating traits of Peribacillus simplex M1 (P. simplex M1) isolates from maize. One endophytic bacterial isolate, named P. simplex M1, was selected from the roots of maize grown in saline-alkali soil. The P. simplex M1 genome sequence analysis of the bacteria with a length of 5.8 Mbp includes about 700 genes that promote growth and 16 antioxidant activity genes that alleviate saline and alkaline stress. P. simplex M1 can grow below 400 mM NaHCO3 on the LB culture medium; The isolate displayed multiple plant growth-stimulating features, such as nitrogen fixation, produced indole-3-acetic acid (IAA), and siderophore production. This isolate had a positive effect on the resistance to salt of maize in addition to the growth. P. simplex M1 significantly promoted seed germination by enhancing seed vigor in maize whether under normal growth or NaHCO3 stress conditions. The seeds with NaHCO3 treatment exhibited higher reactive oxygen species (ROS) levels than the maize in P. simplex M1 inoculant on maize. P. simplex M1 can colonize the roots of maize. The P. simplex M1 inoculant plant increased chlorophyll in leaves, stimulated root and leaf growth, increased the number of lateral roots and root dry weight, increased the length and width of the blades, and dry weight of the blades. The application of inoculants can significantly reduce the content of malondialdehyde (MDA) and increase the activity of plant antioxidant enzymes (Catalase (CAT), Superoxide Dismutase (SOD), and Peroxidase (POD)), which may thereby improve maize resistance to saline and alkaline stress. Conclusion: P. simplex M1 isolate belongs to plant growth-promoting bacteria by having high nitrogen concentration, indoleacetic acid (IAA), and siderophore, and reducing the content of ROS through the antioxidant system to alleviate salt alkali stress. This study presents the potential application of P. simplex M1 as a biological inoculant to promote plant growth and mitigate the saline and alkaline effects of maize and other crops.
Keywords: Peribacillus simplex; endophytic bacteria; maize; plant growth promote; saline and alkaline stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Physiological and genomic insights into a psychrotrophic drought-tolerant bacterial consortium for crop improvement in cold, semiarid regions.Microbiol Res. 2024 Sep;286:127818. doi: 10.1016/j.micres.2024.127818. Epub 2024 Jun 26. Microbiol Res. 2024. PMID: 38970906
-
Seed endophytic bacterium Lysinibacillus sp. (ZM1) from maize (Zea mays L.) shapes its root architecture through modulation of auxin biosynthesis and nitrogen metabolism.Plant Physiol Biochem. 2024 Jul;212:108731. doi: 10.1016/j.plaphy.2024.108731. Epub 2024 May 15. Plant Physiol Biochem. 2024. PMID: 38761545
-
Salt-alkali-tolerant growth-promoting Streptomyces sp. Jrh8-9 enhances alfalfa growth and resilience under saline-alkali stress through integrated modulation of photosynthesis, antioxidant defense, and hormone signaling.Microbiol Res. 2025 Jul;296:128158. doi: 10.1016/j.micres.2025.128158. Epub 2025 Mar 28. Microbiol Res. 2025. PMID: 40164013
-
Advances in deciphering the mechanisms of salt tolerance in Maize.Plant Signal Behav. 2025 Dec;20(1):2479513. doi: 10.1080/15592324.2025.2479513. Epub 2025 Mar 18. Plant Signal Behav. 2025. PMID: 40098499 Free PMC article. Review.
-
Alkaline tolerance in plants: The AT1 gene and beyond.J Plant Physiol. 2024 Dec;303:154373. doi: 10.1016/j.jplph.2024.154373. Epub 2024 Oct 21. J Plant Physiol. 2024. PMID: 39454297 Review.
Cited by
-
The Plant Growth-Promoting Bacterium Bacillus cereus LpBc-47 Can Alleviate the Damage of Saline-Alkali Stress to Lilium pumilum.Microorganisms. 2025 May 28;13(6):1248. doi: 10.3390/microorganisms13061248. Microorganisms. 2025. PMID: 40572136 Free PMC article.
References
-
- Srivastava S., Bist V., Srivastava S., Singh P.C., Trivedi P.K., Asif M.H., Chauhan P.S., Nautiyal C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016;7:587. doi: 10.3389/fpls.2016.00587. - DOI - PMC - PubMed
-
- Ma Y., Tashpolat N. Current status and development trend of soil salinity monitoring research in China. Sustainability. 2023;15:5874. doi: 10.3390/su15075874. - DOI
MeSH terms
Substances
Grants and funding
- 2021YFD1201001/the National Key Research and Development Program of China
- CX23ZD05, CX23JQ04/the Innovation Project of Heilongjiang Academy of Agricultural Sciences
- [2023] 1197/the Heilongjiang Provence Agriculture Research System-Ecological Agri-culture
- LJGXCG2023-036/Heilongjiang Province "Double First Class" Discipline Collaborative Innova-tion Achievement Project
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

