Developmental and Molecular Effects of C-Type Natriuretic Peptide Supplementation in In Vitro Culture of Bovine Embryos
- PMID: 39456721
- PMCID: PMC11507445
- DOI: 10.3390/ijms252010938
Developmental and Molecular Effects of C-Type Natriuretic Peptide Supplementation in In Vitro Culture of Bovine Embryos
Abstract
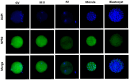
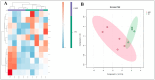
The use of C-type natriuretic peptide (CNP) in the interaction with the oocyte and in the temporary postponement of spontaneous meiosis resumption has already been well described. However, its action in pre-implantation developmental-stage embryos is yet to be understood. Thus, our study aimed to detect the presence of the canonical CNP receptor (natriuretic peptide receptor, NPR2) in germinal vesicle (GV)-, metaphase II (MII)-, presumptive zygote (PZ)-, morula (MO)-, and blastocyst (BL)-stage embryos and, later, to observe possible modulations on the embryos when co-cultured with CNP. In Experiment I, we detected and quantified NPR2 on the abovementioned embryo stages. Further, in Experiment II, we intended to test different concentrations (100, 200, or 400 nM of CNP) at different times of inclusion in the in vitro culture (IVC; inclusion from the beginning, i.e., day 1, or from day 5). In Experiment III, 400 nM of CNP was used on day 1 (D1) in the IVC, which was not demonstrated to be embryotoxic, and it showed potentially promising results in the blastocyst production rate when compared to the control. Thus, we analyzed the embryonic development rates of bovine embryos (D7) and hatching kinetics (D7, D8, and D9). Subsequently, morula and blastocyst were collected and evaluated for transcript abundance of their competence and quality (apoptosis, oxidative stress, proliferation, and differentiation) and lipid metabolism. Differences with probabilities less than p < 0.05, and/or fold change (FC) > 1.5, were considered significant. We demonstrate the presence of NPR2 until the blastocyst development stage, when there was a significant decrease in membrane receptors. There was no statistical difference in the production rate after co-culture with 400 nM CNP. However, when we evaluated the abundance of morula transcripts, there was an upregulated transcription in ADCY6 (p = 0.057) and downregulated transcripts in BMP15 (p = 0.013), ACAT1 (p = 0.040), and CASP3 (p = 0.082). In addition, there was a total of 12 transcriptions in morula that presented variation FC > 1.5. In blastocysts, the treatment with CNP induced upregulation in BID, CASP3, SOX2, and HSPA5 transcripts and downregulation in BDNF, NLRP5, ELOVL1, ELOVL4, IGFBP4, and FDX1 transcripts (FC > 1.5). Thus, our study identified and quantified the presence of NPR2 in bovine pre-implantation embryos. Furthermore, 400 nM of CNP in IVC, a concentration not previously described in the literature, modulated some transcripts related to embryonic metabolism, and this was not embryotoxic morphologically.
Keywords: C-type natriuretic peptide; NPR2; cattle; embryonic metabolism; transcript abundance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Modulation of lipid composition and gene expression by CNP supplementation in in vitro cultured bovine embryos.Sci Rep. 2025 Jul 2;15(1):22972. doi: 10.1038/s41598-025-07453-0. Sci Rep. 2025. PMID: 40593265 Free PMC article.
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Expression and localization of Npr2 in mouse oocytes and pre-implantation embryos.Biotech Histochem. 2019 Jul;94(5):320-324. doi: 10.1080/10520295.2019.1566570. Epub 2019 Feb 7. Biotech Histochem. 2019. PMID: 30729815
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
-
Recent insights into the in vitro culture systems for mammalian embryos.Curr Opin Genet Dev. 2025 Apr;91:102309. doi: 10.1016/j.gde.2025.102309. Epub 2025 Jan 18. Curr Opin Genet Dev. 2025. PMID: 39827579 Review.
Cited by
-
Modulation of lipid composition and gene expression by CNP supplementation in in vitro cultured bovine embryos.Sci Rep. 2025 Jul 2;15(1):22972. doi: 10.1038/s41598-025-07453-0. Sci Rep. 2025. PMID: 40593265 Free PMC article.
References
-
- Franciosi F., Coticchio G., Lodde V., Tessaro I., Modina S.C., Fadini R., Dal Canto M., Renzini M.M., Albertini D.F., Luciano A.M. Natriuretic Peptide Precursor C Delays Meiotic Resumption and Sustains Gap Junction-Mediated Communication in Bovine Cumulus-Enclosed Oocytes1. Biol. Reprod. 2014;91:61. doi: 10.1095/biolreprod.114.118869. - DOI - PubMed
-
- Botigelli R.C., Razza E.M., Pioltine E.M., Fontes P.K., Schwarz K.R.L., Leal C.L.V., Nogueira M.F.G. Supplementing in Vitro Embryo Production Media by NPPC and Sildenafil Affect the Cytoplasmic Lipid Content and Gene Expression of Bovine Cumulus-Oocyte Complexes and Embryos. Reprod. Biol. 2018;18:66–75. doi: 10.1016/j.repbio.2018.01.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

