Advances and Challenges of Bioassembly Strategies in Neurovascular In Vitro Modeling: An Overview of Current Technologies with a Focus on Three-Dimensional Bioprinting
- PMID: 39456783
- PMCID: PMC11506837
- DOI: 10.3390/ijms252011000
Advances and Challenges of Bioassembly Strategies in Neurovascular In Vitro Modeling: An Overview of Current Technologies with a Focus on Three-Dimensional Bioprinting
Abstract
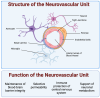
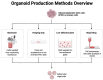
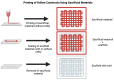
Bioassembly encompasses various techniques such as bioprinting, microfluidics, organoids, and self-assembly, enabling advances in tissue engineering and regenerative medicine. Advancements in bioassembly technologies have enabled the precise arrangement and integration of various cell types to more closely mimic the complexity functionality of the neurovascular unit (NVU) and that of other biodiverse multicellular tissue structures. In this context, bioprinting offers the ability to deposit cells in a spatially controlled manner, facilitating the construction of interconnected networks. Scaffold-based assembly strategies provide structural support and guidance cues for cell growth, enabling the formation of complex bio-constructs. Self-assembly approaches utilize the inherent properties of cells to drive the spontaneous organization and interaction of neuronal and vascular components. However, recreating the intricate microarchitecture and functional characteristics of a tissue/organ poses additional challenges. Advancements in bioassembly techniques and materials hold great promise for addressing these challenges. The further refinement of bioprinting technologies, such as improved resolution and the incorporation of multiple cell types, can enhance the accuracy and complexity of the biological constructs; however, developing bioinks that support the growth of cells, viability, and functionality while maintaining compatibility with the bioassembly process remains an unmet need in the field, and further advancements in the design of bioactive and biodegradable scaffolds will aid in controlling cell adhesion, differentiation, and vascularization within the engineered tissue. Additionally, integrating advanced imaging and analytical techniques can provide real-time monitoring and characterization of bioassembly, aiding in quality control and optimization. While challenges remain, ongoing research and technological advancements propel the field forward, paving the way for transformative developments in neurovascular research and tissue engineering. This work provides an overview of the advancements, challenges, and future perspectives in bioassembly for fabricating neurovascular constructs with an add-on focus on bioprinting technologies.
Keywords: alternatives; biomaterial; bioprinting; blood-brain barrier; cells; in vitro; matrix; neurovascular.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

