Circulatory Indicators of Lipid Peroxidation, the Driver of Ferroptosis, Reflect Differences between Relapsing-Remitting and Progressive Multiple Sclerosis
- PMID: 39456806
- PMCID: PMC11507982
- DOI: 10.3390/ijms252011024
Circulatory Indicators of Lipid Peroxidation, the Driver of Ferroptosis, Reflect Differences between Relapsing-Remitting and Progressive Multiple Sclerosis
Abstract
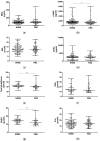
Ferroptosis, a lipid peroxidation- and iron-mediated type of regulated cell death, relates to both neuroinflammation, which is common in relapsing-remitting multiple sclerosis (RRMS), and neurodegeneration, which is prevalent in progressive (P)MS. Currently, findings related to the molecular markers proposed in this paper in patients are scarce. We analyzed circulatory molecular indicators of the main ferroptosis-related processes, comprising lipid peroxidation (malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and hexanoyl-lysine adduct (HEL)), glutathione-related antioxidant defense (total glutathione (reduced (GSH) and oxidized (GSSG)) and glutathione peroxidase 4 (GPX4)), and iron metabolism (iron, transferrin and ferritin) to estimate their contributions to the clinical manifestation of MS and differences between RRMS and PMS disease course. In 153 patients with RRMS and 69 with PMS, plasma/serum lipid peroxidation indicators and glutathione were quantified using ELISA and colorimetric reactions, respectively. Iron serum concentrations were determined using spectrophotometry, and transferrin and ferritin were determined using immunoturbidimetry. Compared to those with RRMS, patients with PMS had decreased 4-HNE (median, 1368.42 vs. 1580.17 pg/mL; p = 0.03). Interactive effects of MS course (RRMS/PMS) and disease-modifying therapy status on MDA (p = 0.009) and HEL (p = 0.02) levels were detected. In addition, the interaction of disease course and self-reported fatigue revealed significant impacts on 4-HNE levels (p = 0.01) and the GSH/GSSG ratio (p = 0.04). The results also show an association of MS course (p = 0.03) and EDSS (p = 0.04) with GSH levels. No significant changes were observed in the serum concentrations of iron metabolism indicators between the two patient groups (p > 0.05). We suggest circulatory 4-HNE as an important parameter related to differences between RRMS and PMS. Significant interactions of MS course and other clinically relevant parameters with changes in redox processes associated with ferroptosis support the further investigation of MS with a larger sample while taking into account both circulatory and central nervous system estimation.
Keywords: ferroptosis; glutathione antioxidant defense; iron metabolism; lipid peroxidation; multiple sclerosis; progression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

