Harmony in Healing: Investigating Platelet-Rich Plasma Activation during Acetylsalicylic Acid Treatment
- PMID: 39456821
- PMCID: PMC11508067
- DOI: 10.3390/ijms252011037
Harmony in Healing: Investigating Platelet-Rich Plasma Activation during Acetylsalicylic Acid Treatment
Abstract
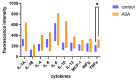
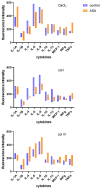
Platelet-rich plasma (PRP) therapy holds promise for treating various clinical conditions. The activation process is crucial in releasing growth factors and cytokines from platelets, enhancing the therapeutic properties of PRP. Standard activation methods involve autologous thrombin or collagen, with variations in efficacy and growth factor release. This study explores the impact of acetylsalicylic acid (ASA), a commonly used antiplatelet drug, on PRP activation. The results indicate that non-activated PRP extracted from the whole blood of ASA-treated patients exhibits increased inflammatory cytokine concentrations, notably TNFa. After activation with autologous thrombin/CaCl2 or collagen IV, the measured fluorescence intensities suggest varying release patterns between treated and non-treated groups. Understanding the influence of ASA on platelet activation holds implications for personalized medicine and optimizing outcomes for individual patients undergoing PRP therapy. This research sheds light on the potential challenges associated with using antiplatelet drugs, emphasizing the need for careful consideration in tailoring PRP-based regenerative therapies.
Keywords: antiplatelet drugs; collagen; inflammatory cytokines; platelet-rich plasma; regenerative therapies; thrombin.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Figures

Similar articles
-
Effects of Aspirin on Growth Factor Release From Freshly Isolated Leukocyte-Rich Platelet-Rich Plasma in Healthy Men: A Prospective Fixed-Sequence Controlled Laboratory Study.Am J Sports Med. 2019 Apr;47(5):1223-1229. doi: 10.1177/0363546519827294. Epub 2019 Mar 19. Am J Sports Med. 2019. PMID: 30888847
-
Normalization of platelet reactivity in clopidogrel-treated subjects.J Thromb Haemost. 2007 Jan;5(1):82-90. doi: 10.1111/j.1538-7836.2006.02245.x. J Thromb Haemost. 2007. PMID: 17239165 Clinical Trial.
-
Modification of Pulsed Electric Field Conditions Results in Distinct Activation Profiles of Platelet-Rich Plasma.PLoS One. 2016 Aug 24;11(8):e0160933. doi: 10.1371/journal.pone.0160933. eCollection 2016. PLoS One. 2016. PMID: 27556645 Free PMC article.
-
PRP in wound healing applications.Platelets. 2021 Feb 17;32(2):189-199. doi: 10.1080/09537104.2020.1849605. Epub 2020 Nov 29. Platelets. 2021. PMID: 33251921 Review.
-
Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing.Int J Mol Sci. 2024 Jul 19;25(14):7914. doi: 10.3390/ijms25147914. Int J Mol Sci. 2024. PMID: 39063156 Free PMC article. Review.
Cited by
-
Individualized parameters for mechanical ventilation during thoracic operations: Optimizing respiratory support.Can J Respir Ther. 2025 May 13;61:117-127. doi: 10.29390/001c.137289. eCollection 2025. Can J Respir Ther. 2025. PMID: 40475022 Free PMC article.
References
-
- Frelinger A.L., Gerrits A.J., Neculaes V.B., Gremmel T., Torres A.S., Caiafa A., Carmichael S.L., Michelson A.D. Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology. PLoS ONE. 2018;13:e0203557. doi: 10.1371/journal.pone.0203557. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

