Serine Hydroxymethyltransferase 2 Deficiency in the Hematopoietic System Disrupts Erythropoiesis and Induces Anemia in Murine Models
- PMID: 39456851
- PMCID: PMC11508403
- DOI: 10.3390/ijms252011072
Serine Hydroxymethyltransferase 2 Deficiency in the Hematopoietic System Disrupts Erythropoiesis and Induces Anemia in Murine Models
Abstract
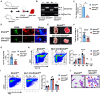
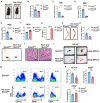
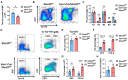
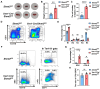
Serine and folate metabolism play critical roles in erythroid development in both embryonic and adult mice; however, the precise roles of these metabolic pathways in erythropoiesis and the pathophysiology of anemia remain inadequately characterized in the literature. To delineate the contributions of serine and folate metabolism to erythroid differentiation, we focused on serine hydroxymethyltransferase 2 (SHMT2), a key regulatory enzyme within these metabolic pathways. Using gene-editing techniques, we created fetal and adult mouse models with targeted deletion of Shmt2 in the hematopoietic system. Our findings demonstrated that the deletion of Shmt2 within the hematopoietic system led to the distinctive anemia phenotype in both fetal and adult mice. Detailed progression analysis of anemia revealed that Shmt2 deletion exerts stage-specific effects on the development and maturation of erythroid cells. Specifically, Shmt2 deficiency promoted erythroid differentiation in the R2 (CD71+ Ter119-) cell population residing in the bone marrow while concurrently inhibiting the proliferation and erythroid differentiation of the R3 (CD71+ Ter119+) cell population. This disruption resulted in developmental arrest at the R3 stage, significantly contributing to the anemia phenotype observed in the models. This study elucidates the critical role of Shmt2 in erythroid development within the hematopoietic system, highlighting the underlying mechanisms of erythroid developmental arrest associated with Shmt2 loss.
Keywords: Vav1-Cre; anemia; bone marrow; erythroid differentiation; red blood cell; serine hydroxymethyltransferase 2.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ashorobi D., Munakomi S. Myelophthisic Anemia. StatPearls Publishing; Treasure Island, FL, USA: 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

