Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery
- PMID: 39456873
- PMCID: PMC11508290
- DOI: 10.3390/ijms252011092
Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery
Abstract
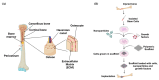
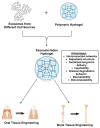
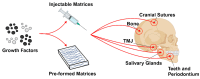
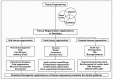
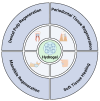
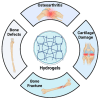
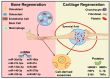
Mineralization is a key biological process that is required for the development and repair of tissues such as teeth, bone and cartilage. Exosomes (Exo) are a subset of extracellular vesicles (~50-150 nm) that are secreted by cells and contain genetic material, proteins, lipids, nucleic acids, and other biological substances that have been extensively researched for bone and oral tissue regeneration. However, Exo-free biomaterials or exosome treatments exhibit poor bioavailability and lack controlled release mechanisms at the target site during tissue regeneration. By encapsulating the Exos into biomaterials like hydrogels, these disadvantages can be mitigated. Several tissue engineering approaches, such as those for wound healing processes in diabetes mellitus, treatment of osteoarthritis (OA) and cartilage degeneration, repair of intervertebral disc degeneration, and cardiovascular diseases, etc., have been exploited to deliver exosomes containing a variety of therapeutic and diagnostic cargos to target tissues. Despite the significant efficacy of Exo-laden hydrogels, their use in mineralized tissues, such as oral and bone tissue, is very sparse. This review aims to explore and summarize the literature related to the therapeutic potential of hydrogel-encapsulated exosomes for bone and oral tissue engineering and provides insight and practical procedures for the development of future clinical techniques.
Keywords: biomaterials; bone tissue engineering; exosomes; hydrogels; mineralized tissues; oral tissue engineering; regenerative medicine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Zhang M.M., Xu S.X., Wang R.Y., Che Y.A., Han C.C., Feng W., Wang C.W., Zhao W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023;162:157–178. doi: 10.1016/j.jmst.2023.04.015. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

