Polydatin Prevents Electron Transport Chain Dysfunction and ROS Overproduction Paralleled by an Improvement in Lipid Peroxidation and Cardiolipin Levels in Iron-Overloaded Rat Liver Mitochondria
- PMID: 39456885
- PMCID: PMC11508176
- DOI: 10.3390/ijms252011104
Polydatin Prevents Electron Transport Chain Dysfunction and ROS Overproduction Paralleled by an Improvement in Lipid Peroxidation and Cardiolipin Levels in Iron-Overloaded Rat Liver Mitochondria
Abstract
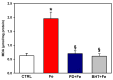
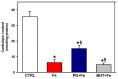
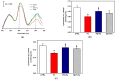
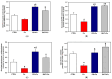
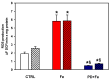
Increased intramitochondrial free iron is a key feature of various liver diseases, leading to oxidative stress, mitochondrial dysfunction, and liver damage. Polydatin is a polyphenol with a hepatoprotective effect, which has been attributed to its ability to enhance mitochondrial oxidative metabolism and antioxidant defenses, thereby inhibiting reactive oxygen species (ROS) dependent cellular damage processes and liver diseases. However, it has not been explored whether polydatin is able to exert its effects by protecting the phospholipid cardiolipin against damage from excess iron. Cardiolipin maintains the integrity and function of electron transport chain (ETC) complexes and keeps cytochrome c bound to mitochondria, avoiding uncontrolled apoptosis. Therefore, the effect of polydatin on oxidative lipid damage, ETC activity, cytochrome levels, and ROS production was explored in iron-exposed rat liver mitochondria. Fe2+ increased lipid peroxidation, decreased cardiolipin and cytochromes c + c1 and aa3 levels, inhibited ETC complex activities, and dramatically increased ROS production. Preincubation with polydatin prevented all these effects to a variable degree. These results suggest that the hepatoprotective mechanism of polydatin involves the attenuation of free radical production by iron, which enhances cardiolipin levels by counteracting membrane lipid peroxidation. This prevents the loss of cytochromes, improves ETC function, and decreases mitochondrial ROS production.
Keywords: Fe2+; cytochromes; free radicals; hydroxyl radical; iron overload; liver disease; mitochondrial function; oxidative stress; piceid; respiratory chain.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Szalárdy L., Zádori D., Klivényi P., Toldi J., Vécsei L. Electron Transport Disturbances and Neurodegeneration: From Albert Szent-Györgyi’s Concept (Szeged) till Novel Approaches to Boost Mitochondrial Bioenergetics. Oxid. Med. Cell Longev. 2015;2015:498401. doi: 10.1155/2015/498401. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

