Bioaccumulation Rate of Non-Biodegradable Polystyrene Microplastics in Human Epithelial Cell Lines
- PMID: 39456886
- PMCID: PMC11508641
- DOI: 10.3390/ijms252011101
Bioaccumulation Rate of Non-Biodegradable Polystyrene Microplastics in Human Epithelial Cell Lines
Abstract
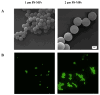
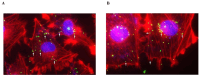
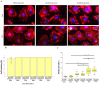
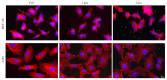
Environment plastic accumulation has been attracting the attention of both political and scientific communities, who wish to reduce global pollution. Plastic items have been detected everywhere, from oceans to the air, raising concerns about the fate of plastics within organisms. Leaked plastics are ingested by animals, entering the food chain and eventually reaching humans. Although a lot of studies focused on the evaluation of plastic particles in the environment and living organisms have already been published, the behavior of plastic at the cellular level is still missing. Here, we analyzed the bioaccumulation and extrusion trend of two differently sized plastic particles (1 and 2 µm), testing them on three human epithelial cell lines (liver, lung, and gut) that represent epithelial sites mainly exposed to plastic. A different behavior was detected, and the major plastic uptake was shown by liver cells, where the 1 µm beads accumulated with a dose-dependent profile. Moreover, a 60% reduction in the content of 1 µm particles in cells was evaluated after plastic removal. Finally, the viability and proliferation of the three human cell lines were not significantly affected by both the 1 and 2 µm beads, suggesting that cells might have a defense mechanism against plastic exposure risk.
Keywords: bioaccumulation; cell survival; microplastic; polystyrene; release.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
-
Plastic pollution and potential solutions.Sci Prog. 2018 Sep 1;101(3):207-260. doi: 10.3184/003685018X15294876706211. Epub 2018 Jul 19. Sci Prog. 2018. PMID: 30025551 Free PMC article. Review.
-
Quantification of Polystyrene Uptake by Different Cell Lines Using Fluorescence Microscopy and Label-Free Visualization of Intracellular Polystyrene Particles by Raman Microspectroscopic Imaging.Cells. 2024 Mar 5;13(5):454. doi: 10.3390/cells13050454. Cells. 2024. PMID: 38474417 Free PMC article.
-
Nano-scale and micron-scale plastics amplify the bioaccumulation of benzophenone-3 and ciprofloxacin, as well as their co-exposure effect on disturbing the antioxidant defense system in mussels, Perna viridis.Environ Pollut. 2024 Apr 1;346:123547. doi: 10.1016/j.envpol.2024.123547. Epub 2024 Feb 20. Environ Pollut. 2024. PMID: 38387549
-
Bioaccumulation of polystyrene microplastics and changes in antioxidant and AChE pattern in a freshwater snail (Filopaludina bengalensis) from river Ganga.Aquat Toxicol. 2023 Oct;263:106697. doi: 10.1016/j.aquatox.2023.106697. Epub 2023 Sep 15. Aquat Toxicol. 2023. PMID: 37774668
Cited by
-
Intratracheal Administration of Polystyrene Micro(nano)plastics with a Mixed Particle Size Promote Pulmonary Fibrosis in Rats by Activating TGF-β1 Signaling and Destabilizing Mitochondrial Dynamics and Mitophagy in a Dose- and Time-Dependent Manner.Toxics. 2025 Jun 9;13(6):487. doi: 10.3390/toxics13060487. Toxics. 2025. PMID: 40559960 Free PMC article.
References
-
- Hollman P. Microplastics and Nanoplastics in Food—An Emerging Issue. [(accessed on 8 March 2024)]. Available online: www.efsa.europa.eu/en/news/microplastics-and-nanoplastics-food-emerging-....
-
- Mkuye R., Gong S., Zhao L., Masanja F., Ndandala C., Bubelwa E., Yang C., Deng Y. Effects of microplastics on physiological performance of marine bivalves, potential impacts, and enlightening the future based on a comparative study. Sci. Total Environ. 2022;838:155933. doi: 10.1016/j.scitotenv.2022.155933. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

