Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral-Gut Axis
- PMID: 39456922
- PMCID: PMC11508520
- DOI: 10.3390/ijms252011141
Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral-Gut Axis
Abstract
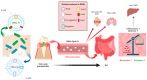
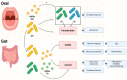
Oral pathobionts are essential in instigating local inflammation within the oral cavity and contribute to the pathogenesis of diseases in the gastrointestinal tract and other distant organs. Among the Gram-negative pathobionts, Porphyromonas gingivalis and Fusobacterium nucleatum emerge as critical drivers of periodontitis, exerting their influence not only locally but also as inducers of gut dysbiosis, intestinal disturbances, and systemic ailments. This dual impact is facilitated by their ectopic colonization of the intestinal mucosa and the subsequent mediation of distal systemic effects by releasing outer membrane vesicles (OMVs) into circulation. This review elucidates the principal components of oral pathobiont-derived OMVs implicated in disease pathogenesis within the oral-gut axis, detailing virulence factors that OMVs carry and their interactions with host epithelial and immune cells, both in vitro and in vivo. Additionally, we shed light on the less acknowledged interplay between oral pathobionts and the gut commensal Akkermansia muciniphila, which can directly impede oral pathobionts' growth and modulate bacterial gene expression. Notably, OMVs derived from A. muciniphila emerge as promoters of anti-inflammatory effects within the gastrointestinal and distant tissues. Consequently, we explore the potential of A. muciniphila-derived OMVs to interact with oral pathobionts and prevent disease in the oral-gut axis.
Keywords: OMVs; dysbiosis; inflammation; innate immunity; oral–gut axis; pathobionts; systemic disease; virulence factors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

