The D75N and P161S Mutations in the C0-C2 Fragment of cMyBP-C Associated with Hypertrophic Cardiomyopathy Disturb the Thin Filament Activation, Nucleotide Exchange in Myosin, and Actin-Myosin Interaction
- PMID: 39456977
- PMCID: PMC11508426
- DOI: 10.3390/ijms252011195
The D75N and P161S Mutations in the C0-C2 Fragment of cMyBP-C Associated with Hypertrophic Cardiomyopathy Disturb the Thin Filament Activation, Nucleotide Exchange in Myosin, and Actin-Myosin Interaction
Abstract
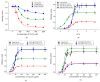
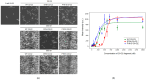
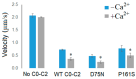
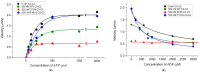
About half of the mutations that lead to hypertrophic cardiomyopathy (HCM) occur in the MYBPC3 gene. However, the molecular mechanisms of pathogenicity of point mutations in cardiac myosin-binding protein C (cMyBP-C) remain poorly understood. In this study, we examined the effects of the D75N and P161S substitutions in the C0 and C1 domains of cMyBP-C on the structural and functional properties of the C0-C1-m-C2 fragment (C0-C2). Differential scanning calorimetry revealed that these mutations disorder the tertiary structure of the C0-C2 molecule. Functionally, the D75N mutation reduced the maximum sliding velocity of regulated thin filaments in an in vitro motility assay, while the P161S mutation increased it. Both mutations significantly reduced the calcium sensitivity of the actin-myosin interaction and impaired thin filament activation by cross-bridges. D75N and P161S C0-C2 fragments substantially decreased the sliding velocity of the F-actin-tropomyosin filament. ADP dose-dependently reduced filament sliding velocity in the presence of WT and P161S fragments, but the velocity remained unchanged with the D75N fragment. We suppose that the D75N mutation alters nucleotide exchange kinetics by decreasing ADP affinity to the ATPase pocket and slowing the myosin cycle. Our molecular dynamics simulations mean that the D75N mutation affects myosin S1 function. Both mutations impair cardiac contractility by disrupting thin filament activation. The results offer new insights into the HCM pathogenesis caused by missense mutations in N-terminal domains of cMyBP-C, highlighting the distinct effects of D75N and P161S mutations on cardiac contractile function.
Keywords: actin–myosin interaction; cardiac myosin-binding protein C; differential scanning calorimetry; hypertrophic cardiomyopathy mutations; in vitro motility assay; thin filament activation.
Conflict of interest statement
Authors Natalia S. Ryabkova and Ivan A. Katrukha were employed by the company HyTest Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ho C.Y., Carlsen C., Thune J.J., Havndrup O., Bundgaard H., Farrohi F., Rivero J., Cirino A.L., Andersen P.S., Christiansen M., et al. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 2009;2:314–321. doi: 10.1161/CIRCGENETICS.109.862128. - DOI - PMC - PubMed
-
- Ho C.Y., Sweitzer N.K., McDonough B., Maron B.J., Casey S.A., Seidman J.G., Seidman C.E., Solomon S.D. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105:2992–2997. doi: 10.1161/01.CIR.0000019070.70491.6D. - DOI - PubMed
-
- Witjas-Paalberends E.R., Ferrara C., Scellini B., Piroddi N., Montag J., Tesi C., Stienen G.J., Michels M., Ho C.Y., Kraft T., et al. Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. J. Physiol. 2014;592:3257–3272. doi: 10.1113/jphysiol.2014.274571. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

