Human iPSCs from Aged Donors Retain Their Mitochondrial Aging Signature
- PMID: 39456998
- PMCID: PMC11508692
- DOI: 10.3390/ijms252011199
Human iPSCs from Aged Donors Retain Their Mitochondrial Aging Signature
Abstract
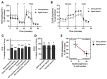
Aging represents the leading risk factor for developing neurodegenerative disorders. One of the nine hallmarks of aging is mitochondrial dysfunction. Age-related mitochondrial alterations have been shown to affect mitochondrial energy metabolism, reduction-oxidation homeostasis, and mitochondrial dynamics. Previous reports have shown that induced pluripotent stem cells (iPSCs) from aged donors do not keep the aging signature at the transcriptomic level. However, not all aspects of aging have been investigated, and especially not the mitochondria-related aging signature. Therefore, the present study compared the mitochondrial function in iPSCs from healthy aged donors compared to those of young donors. We addressed whether aged iPSCs may be used as drug-screening models of "aging in a dish" to identify therapies alleviating mitochondria aging. Compared to iPSCs from young donors, we demonstrate that iPSCs from aged donors show impaired mitochondrial bioenergetics and exhibit a rise in reactive oxygen species generation. Furthermore, aged iPSCs present a lower mitochondrial mass and alterations in the morphology of the mitochondrial network when compared to iPSCs from young donors. This study provides the first evidence that the aging phenotype is present at the mitochondrial level in iPSCs from aged donors, ranging from bioenergetics to mitochondrial network morphology. This model might be used to screen mitochondria-targeting drugs to promote healthy aging at the mitochondrial level.
Keywords: aging; bioenergetics; human-induced pluripotent stem cells; mitochondria; mitochondrial morphology; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 31003A-179294/SNSF_/Swiss National Science Foundation/Switzerland
- 18C143/Novartis Foundation for Medical Research
- 115439/Innovative Medicines Initiative Joint Undertaking
- FP7/2007-2013/European Union's Seventh Framework Programme
- not applicable/European Federation of Pharmaceutical Industries and Associations (EFPIA)
LinkOut - more resources
Full Text Sources
Medical

