Targeted Drug Screening Leveraging Senescence-Induced T-Cell Exhaustion Signatures in Hepatocellular Carcinoma
- PMID: 39457014
- PMCID: PMC11508728
- DOI: 10.3390/ijms252011232
Targeted Drug Screening Leveraging Senescence-Induced T-Cell Exhaustion Signatures in Hepatocellular Carcinoma
Abstract
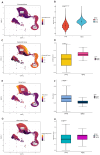
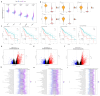
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and a leading cause of cancer-related mortality globally, with most patients diagnosed at advanced stages and facing limited early treatment options. This study aimed to identify characteristic genes associated with T-cell exhaustion due to senescence in hepatocellular carcinoma patients, elucidating the interplay between senescence and T-cell exhaustion. We constructed prognostic models based on five signature genes (ENO1, STMN1, PRDX1, RAN, and RANBP1) linked to T-cell exhaustion, utilizing elastic net regression. The findings indicate that increased expression of ENO1 in T cells may contribute to T-cell exhaustion and Treg infiltration in hepatocellular carcinoma. Furthermore, molecular docking was employed to screen small molecule compounds that target the anti-tumor effects of these exhaustion-related genes. This study provides crucial insights into the diagnosis and treatment of hepatocellular carcinoma, establishing a strong foundation for the development of predictive biomarkers and therapeutic targets for affected patients.
Keywords: cellular senescence; combined targeted therapy; hepatocellular carcinoma; machine learning; single-cell RNA-seq; t cell exhaustion.
Conflict of interest statement
The authors declare that they have no competing interests in this section.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

