The Large GTPase Guanylate-Binding Protein-1 (GBP-1) Promotes Mitochondrial Fission in Glioblastoma
- PMID: 39457021
- PMCID: PMC11508455
- DOI: 10.3390/ijms252011236
The Large GTPase Guanylate-Binding Protein-1 (GBP-1) Promotes Mitochondrial Fission in Glioblastoma
Abstract
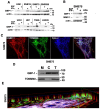
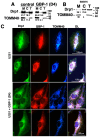
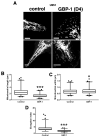
Glioblastomas (aka Glioblastoma multiformes (GBMs)) are the most deadly of the adult brain tumors. Even with aggressive treatment, the prognosis is extremely poor. The large GTPase Guanylate-Binding Protein-1 (GBP-1) contributes to the poor prognosis of GBM by promoting migration and invasion. GBP-1 is substantially localized to the cytosolic side of the outer membrane of mitochondria in GBM cells. Because mitochondrial dynamics, particularly mitochondrial fission, can drive cell migration and invasion, the potential interactions between GBP-1 and mitochondrial dynamin-related protein 1 (Drp1) were explored. Drp1 is the major driver of mitochondrial fission. While GBP-1 and Drp1 both had punctate distributions within the cytoplasm and localized to regions of the cytoplasmic side of the plasma membrane of GBM cells, the proteins were only molecularly co-localized at the mitochondria. Subcellular fractionation showed that the presence of elevated GBP-1 promoted the movement of Drp1 from the cytosol to the mitochondria. The migration of U251 cells treated with the Drp1 inhibitor, Mdivi-1, was less inhibited in the cells with elevated GBP-1. Elevated GBP-1 in GBM cells resulted in shorter and wider mitochondria, most likely from mitochondrial fission. Mitochondrial fission can drive several important cellular processes, including cell migration, invasion, and metastasis.
Keywords: Dynamin-like Proteins (DLPs); Epidermal Growth Factor Receptor (EGFR); Guanylate-Binding Protein-1 (GBP-1); Translocase of Outer Mitochondrial Membrane 40 (TOMM40); glioblastoma multiforme (GBM); immunofluorescence; mitochondrial dynamin-related protein 1 (Drp1).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Libermann T.A., Nusbaum H.R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M.D., Ullrich A., Schlessinger J. Amplification and overexpression of the EGF receptor gene in primary human glioblastomas. J. Cell Sci. Suppl. 1985;3:161–172. doi: 10.1242/jcs.1985.Supplement_3.16. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

