Downregulation of the GhROD1 Gene Improves Cotton Fiber Fineness by Decreasing Acyl Pool Saturation, Stimulating Small Heat Shock Proteins (sHSPs), and Reducing H2O2 Production
- PMID: 39457024
- PMCID: PMC11509027
- DOI: 10.3390/ijms252011242
Downregulation of the GhROD1 Gene Improves Cotton Fiber Fineness by Decreasing Acyl Pool Saturation, Stimulating Small Heat Shock Proteins (sHSPs), and Reducing H2O2 Production
Abstract
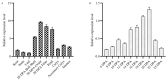
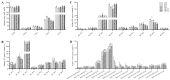
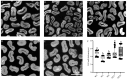
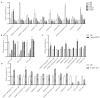
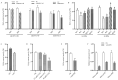
Cotton fiber is one of the most important natural fiber sources in the world, and lipid metabolism plays a critical role in its development. However, the specific role of lipid molecules in fiber development and the impact of fatty acid alterations on fiber quality remain largely unknown. In this study, we demonstrate that the downregulation of GhROD1, a gene encoding phosphatidylcholine diacylglycerol cholinephosphotransferase (PDCT), results in an improvement of fiber fineness. We found that GhROD1 downregulation significantly increases the proportion of linoleic acid (18:2) in cotton fibers, which subsequently upregulates genes encoding small heat shock proteins (sHSPs). This, in turn, reduces H2O2 production, thus delaying secondary wall deposition and leading to finer fibers. Our findings reveal how alterations in linoleic acid influence cellulose synthesis and suggest a potential strategy to improve cotton fiber quality by regulating lipid metabolism pathways.
Keywords: GhROD1; Gossypium hirsutum L.; H2O2; cellulose synthesis; cotton fiber development; linoleic acid; phosphatidylcholine diacylglycerol cholinephosphotransferase (PDCT); small heat shock proteins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Gene expression profile analysis of Ligon lintless-1 (Li1) mutant reveals important genes and pathways in cotton leaf and fiber development.Gene. 2014 Feb 10;535(2):273-85. doi: 10.1016/j.gene.2013.11.017. Epub 2013 Nov 23. Gene. 2014. PMID: 24279997
-
Quantitative proteomics and transcriptomics reveal key metabolic processes associated with cotton fiber initiation.J Proteomics. 2015 Jan 30;114:16-27. doi: 10.1016/j.jprot.2014.10.022. Epub 2014 Nov 8. J Proteomics. 2015. PMID: 25449837
-
The dual functions of WLIM1a in cell elongation and secondary wall formation in developing cotton fibers.Plant Cell. 2013 Nov;25(11):4421-38. doi: 10.1105/tpc.113.116970. Epub 2013 Nov 12. Plant Cell. 2013. PMID: 24220634 Free PMC article.
-
MIXTAs and phytohormones orchestrate cotton fiber development.Curr Opin Plant Biol. 2021 Feb;59:101975. doi: 10.1016/j.pbi.2020.10.007. Epub 2020 Dec 6. Curr Opin Plant Biol. 2021. PMID: 33296746 Review.
-
Molecular Regulation of Cotton Fiber Development: A Review.Int J Mol Sci. 2022 Apr 30;23(9):5004. doi: 10.3390/ijms23095004. Int J Mol Sci. 2022. PMID: 35563394 Free PMC article. Review.
Cited by
-
Cotton under heat stress: a comprehensive review of molecular breeding, genomics, and multi-omics strategies.Front Genet. 2025 Mar 18;16:1553406. doi: 10.3389/fgene.2025.1553406. eCollection 2025. Front Genet. 2025. PMID: 40171219 Free PMC article. Review.
References
-
- Jung J., Maeda M., Chang A., Landivar J., Yeom J., McGinty J. Unmanned aerial system assisted framework for the selection of high yielding cotton genotypes. Comput. Electron. Agric. 2018;152:74–81. doi: 10.1016/j.compag.2018.06.051. - DOI
-
- Khan M.A., Wahid A., Ahmad M., Tahir M.T., Ahmed M., Ahmad S., Hasanuzzaman M. World cotton production and consumption: An overview. In: Ahmad S., Hasanuzzaman M., editors. Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies. Springer; Singapore: 2020. pp. 1–7.
-
- Ohlrogge J., Browse J., Jaworski J., Somerville C. Lipids. In: Buchanan B.B., Gruissem W., Jones R.L., editors. Biochemistry and Molecular Biology of Plants. Wiley; Oxford, UK: 2015. pp. 337–344.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

