Encapsulation of Dexamethasone into mRNA-Lipid Nanoparticles Is a Promising Approach for the Development of Liver-Targeted Anti-Inflammatory Therapies
- PMID: 39457035
- PMCID: PMC11508592
- DOI: 10.3390/ijms252011254
Encapsulation of Dexamethasone into mRNA-Lipid Nanoparticles Is a Promising Approach for the Development of Liver-Targeted Anti-Inflammatory Therapies
Abstract
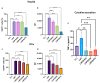
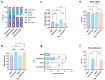
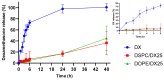
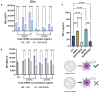
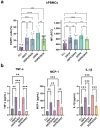
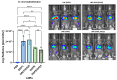
The objective of this study was to develop two lipid nanoparticle (LNP) formulations capable of efficiently expressing a reporter mRNA while co-delivering the anti-inflammatory drug dexamethasone (DX) to reduce inflammatory side effects in protein replacement therapies. Two types of LNPs were developed, in which 25% of cholesterol was replaced by DX. These LNPs contained either 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) or 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) as a helper lipid. The resulting LNPs exhibited high stability, homogeneity, and near-neutral Zeta potentials. SAXS experiments confirmed DX incorporation into the LNP core, with slow in vitro DX release observed over 48 h. The LNPs achieved high mRNA encapsulation efficiency (95-100%) and effectively transfected HepG2 cells, dendritic cells, and hPBMCs. While LNPs increased cytokine release (IL-1β, TNF-α, MCP-1), LNPs-DX significantly reduced cytokine levels, demonstrating enhanced anti-inflammatory properties while maintaining mRNA expression levels. In vivo biodistribution showed predominant liver localization post-intramuscular injection, regardless of the DSPC or DOPE composition. LNPs co-loaded with mRNA and DX are promising candidates for continuous protein replacement. Due to their ability to reduce treatment-related inflammation while maintaining significant mRNA expression levels, these LNPs are perfectly suited for the treatment of liver-related metabolic diseases.
Keywords: anti-inflammatory; cytokines; dendritic cells; dexamethasone; hPBMCs; in vivo biodistribution; lipid nanoparticles; mRNA delivery.
Conflict of interest statement
M.L.C. is currently an employee at BioNTech SE (Mainz, Germany); however, the contributions from M.L.C. were made prior to his employment at BioNTech. The company had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

