The Therapeutic Potential of Adipose-Derived Mesenchymal Stem Cell Secretome in Osteoarthritis: A Comprehensive Study
- PMID: 39457070
- PMCID: PMC11508730
- DOI: 10.3390/ijms252011287
The Therapeutic Potential of Adipose-Derived Mesenchymal Stem Cell Secretome in Osteoarthritis: A Comprehensive Study
Abstract
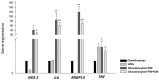
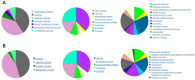
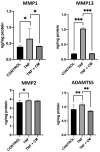
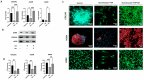
Osteoarthritis (OA) is a degenerative joint disease characterized by cartilage degradation and inflammation. This study investigates the therapeutic potential of secretome derived from adipose tissue mesenchymal stem cells (ASCs) in mitigating inflammation and promoting cartilage repair in an in vitro model of OA. Our in vitro model comprised chondrocytes inflamed with TNF. To assess the therapeutic potential of secretome, inflamed chondrocytes were treated with it and concentrations of pro-inflammatory cytokines, metalloproteinases (MMPs) and extracellular matrix markers were measured. In addition, secretome-treated chondrocytes were subject to a microarray analysis to determine which genes were upregulated and which were downregulated. Treating TNF-inflamed chondrocytes with secretome in vitro inhibits the NF-κB pathway, thereby mediating anti-inflammatory and anti-catabolic effects. Additional protective effects of secretome on cartilage are revealed in the inhibition of hypertrophy markers such as RUNX2 and COL10A1, increased production of COL2A1 and ACAN and upregulation of SOX9. These findings suggest that ASC-derived secretome can effectively reduce inflammation, promote cartilage repair, and maintain chondrocyte phenotype. This study highlights the potential of ASC-derived secretome as a novel, non-cell-based therapeutic approach for OA, offering a promising alternative to current treatments by targeting inflammation and cartilage repair mechanisms.
Keywords: conditioned medium; inflammatory cytokines; mesenchymal stem cells; osteoarthritis; secretome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fernandes J.C., Martel-Pelletier J., Pelletier J.-P. The Role of Cytokines in Osteoarthritis Pathophysiology. Biorheology. 2002;39:237–246. - PubMed
-
- Tetlow L.C., Adlam D.J., Woolley D.E. Matrix Metalloproteinase and Proinflammatory Cytokine Production by Chondrocytes of Human Osteoarthritic Cartilage: Associations with Degenerative Changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

