α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation
- PMID: 39457087
- PMCID: PMC11509036
- DOI: 10.3390/ijms252011305
α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation
Abstract
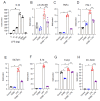
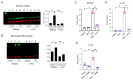
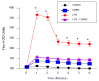
Sepsis remains an important healthcare challenge. The lungs are often affected in sepsis, resulting in acute lung injury characterized by inflammation. Mechanisms involving lipopolysaccharide (LPS) stimulation of toll-like receptor (TLR) signaling with induction of proinflammatory pathways have been implicated in this process. To date, however, studies targeting these pathways have failed to improve outcomes. We have found that LPS may also promote lung injury through the activation of α4 nicotinic acetylcholine receptors (α4 nAChRs) in immune cells. We observed increased expression of α4 nAChRs in human THP-1 monocytic cells exposed to LPS (100 ng/mL, 24 h). We also observed that LPS stimulated the expression of other relevant genes, including tumor necrosis factor-α, interleukin-1β, plasminogen activator inhibitor-1, the solute carrier family 7 member 11, extracellular superoxide dismutase, and transforming growth factor-β1. Of interest, dihydro-β-erythroidine hydrobromide (DHβE), a specific chemical inhibitor of α4 nAChRs, inhibited the LPS-induced expression of these genes. We generated mice with a global knockout mutation of the α4 nAChR subunit in the C57BL/6 background using CRISPR/Cas9 technology. The lungs of these LPS-treated animals demonstrated a reduction in the expression of the above-mentioned genes when compared with the lungs of wild-type animals. In support of the role of oxidative stress, we observed that LPS induced expression of the cystine transporter Slc7a11 in both THP-1 cells and in wild-type mouse lungs. The effects of LPS on THP-1 cells were blocked by the thiol antioxidant N-acetylcysteine and mimicked by redox stress. Importantly, the induction of IL-1β by redox stress was inhibited by the α4 nAChR inhibitor DHβE. Finally, we showed that LPS stimulated calcium influx in THP-1 cells, which was blocked by the α4 nAChR inhibitor. Our observations suggest that LPS promotes lung injury by stimulating redox stress, which activates α4 nAChR signaling and drives proinflammatory cytokine expression.
Keywords: lung injury; monocytic cells; nicotinic receptors; redox stress; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

