Proliferative Diabetic Retinopathy Microenvironment Drives Microglial Polarization and Promotes Angiogenesis and Fibrosis via Cyclooxygenase-2/Prostaglandin E2 Signaling
- PMID: 39457089
- PMCID: PMC11508523
- DOI: 10.3390/ijms252011307
Proliferative Diabetic Retinopathy Microenvironment Drives Microglial Polarization and Promotes Angiogenesis and Fibrosis via Cyclooxygenase-2/Prostaglandin E2 Signaling
Abstract
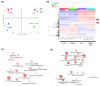
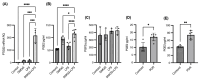
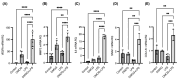
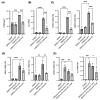
Diabetic retinopathy (DR) is the leading cause of visual impairment, particularly in the proliferative form (proliferative DR [PDR]). The impact of the PDR microenvironment on microglia, which are the resident immune cells in the central nervous system, and the specific pathological changes it may induce remain unclear. This study aimed to investigate the role of microglia in the progression of PDR under hypoxic and inflammatory conditions. We performed a comprehensive gene expression analysis using human-induced pluripotent stem cell-derived microglia under different stimuli (dimethyloxalylglycine (DMOG), lipopolysaccharide (LPS), and DMOG + LPS) to mimic the hypoxic inflammatory environment characteristic of PDR. Principal component analysis revealed distinct gene expression profiles, with 76 genes synergistically upregulated under combined stimulation. Notably, prostaglandin-endoperoxide synthase 2 (encoding cyclooxygenase (COX)-2) exhibited the most pronounced increase, leading to elevated prostaglandin E2 (PGE2) levels and driving pathological angiogenesis and inflammation via the COX-2/PGE2/PGE receptor 2 signaling axis. Additionally, the upregulation of the fibrogenic genes snail family transcriptional repressor 1 and collagen type I alpha 1 chain suggested a role for microglia in fibrosis. These findings underscore the critical involvement of microglia in PDR and suggest that targeting both the angiogenic and fibrotic pathways may present new therapeutic strategies for managing this condition.
Keywords: COX-2; diabetic retinopathy; human microglia; hypoxia; inflammation; neurovascular unit.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Single-cell analysis identifies MKI67+ microglia as drivers of neovascularization in proliferative diabetic retinopathy.J Transl Med. 2025 Mar 11;23(1):310. doi: 10.1186/s12967-025-06320-w. J Transl Med. 2025. PMID: 40069725 Free PMC article.
-
Differential expression of E-type prostanoid receptors 2 and 4 in microglia stimulated with lipopolysaccharide.J Neuroinflammation. 2017 Jan 5;14(1):3. doi: 10.1186/s12974-016-0780-7. J Neuroinflammation. 2017. PMID: 28086956 Free PMC article.
-
Expression of cyclooxygenase-1/-2, microsomal prostaglandin-E synthase-1 and E-prostanoid receptor 2 and regulation of inflammatory mediators by PGE(2) in the amoeboid microglia in hypoxic postnatal rats and murine BV-2 cells.Neuroscience. 2009 Dec 15;164(3):948-62. doi: 10.1016/j.neuroscience.2009.08.044. Epub 2009 Aug 25. Neuroscience. 2009. PMID: 19712723
-
Microglia in retinal angiogenesis and diabetic retinopathy.Angiogenesis. 2024 Aug;27(3):311-331. doi: 10.1007/s10456-024-09911-1. Epub 2024 Apr 2. Angiogenesis. 2024. PMID: 38564108 Free PMC article. Review.
-
Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy.Int J Mol Sci. 2018 Dec 13;19(12):4034. doi: 10.3390/ijms19124034. Int J Mol Sci. 2018. PMID: 30551619 Free PMC article. Review.
Cited by
-
CEP78 regulates the proliferation and angiogenesis of endothelial cells by mediating microtubule stabilization via p150glued.J Mol Histol. 2025 Aug 21;56(5):270. doi: 10.1007/s10735-025-10556-7. J Mol Histol. 2025. PMID: 40839038 No abstract available.
-
EMMPRIN aggravates angiogenesis and blood-retina barrier injury by regulating matrix metalloproteinases in diabetic retinopathy.Diab Vasc Dis Res. 2025 Mar-Apr;22(2):14791641251324556. doi: 10.1177/14791641251324556. Epub 2025 Apr 18. Diab Vasc Dis Res. 2025. PMID: 40251743 Free PMC article.
References
-
- Bressler N.M., Beaulieu W.T., Glassman A.R., Blinder K.J., Bressler S.B., Jampol L.M., Melia M., Wells J.A., 3rd, Diabetic Retinopathy Clinical Research Network Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema with Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018;136:257–269. doi: 10.1001/jamaophthalmol.2017.6565. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

