Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim
- PMID: 39457095
- PMCID: PMC11508328
- DOI: 10.3390/ijms252011314
Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim
Abstract
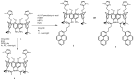
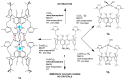
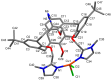
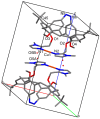
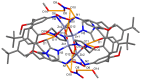
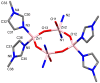
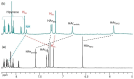
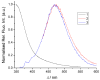
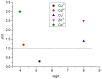
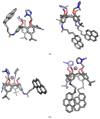
Herein, the presented results show that previously studied DNA/RNA-interacting bis-imidazole-calix[4]arene systems can, in aqueous solutions, efficiently bind a series of biorelevant transition metal cations by coordination with the two imidazole arms at the small rim of their macrocyclic basket. The SCXRD and NMR results structurally characterised the complexes formed by referent bis-imidazole-calix[4]arene with Cu2+ and Zn2+. In solid-state (crystal), the bis-anilino derivative/Cu2+ complex, only upon exposure to the air, undergoes intramolecular dehydrogenative coupling of two neighbouring aniline units, yielding an azo bridge at the large rim of the calix[4]arene basket. In the biorelevant aqueous solution, the comparison of fluorometric titrations of referent calix[4]arene, with its analogues having one or two pyrene units grafted at the opposite (large) rim, revealed moderate-to-strong affinity towards transition metal cations, and, more importantly, a strong impact of pyrene on the binding affinity towards some cations. The pyrene arm(s) significantly diminished the affinity of the calix[4]arene-imidazole ligand towards Cu+ and strongly increased the affinity towards divalent Co2+ and Cd2+ cations. Moreover, the fluorometric response of some studied derivatives was strappingly sensitive to cation type. Since the counter-anion plays only a marginal role, such a change in selectivity is attributed to the intramolecular interaction of pyrene(s) with the calix[4]arene-imidazole system, sterically controlling the metal cation binding site.
Keywords: X-ray structures; calix[4]arene; dehydrogenative coupling; fluorescence; metal coordination; pyrene excimer/exciplex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Electrochemically triggered double translocation of two different metal ions with a ditopic calix[6]arene ligand.J Am Chem Soc. 2010 Mar 31;132(12):4393-8. doi: 10.1021/ja910676z. J Am Chem Soc. 2010. PMID: 20210313
-
Calix[4]arenes with one and two N-linked imidazolium units as precursors of N-heterocyclic carbene complexes. Coordination chemistry and use in Suzuki-Miyaura cross-coupling.Dalton Trans. 2011 Oct 14;40(38):9889-98. doi: 10.1039/c1dt10838g. Epub 2011 Aug 30. Dalton Trans. 2011. PMID: 21879086
-
Accessible gold clusters using calix[4]arene N-heterocyclic carbene and phosphine ligands.Dalton Trans. 2013 Sep 21;42(35):12762-71. doi: 10.1039/c3dt50804h. Dalton Trans. 2013. PMID: 23917776
-
1,3-Diketone Calix[4]arene Derivatives-A New Type of Versatile Ligands for Metal Complexes and Nanoparticles.Molecules. 2021 Feb 24;26(5):1214. doi: 10.3390/molecules26051214. Molecules. 2021. PMID: 33668373 Free PMC article. Review.
-
Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors.Chem Rev. 2011 Aug 10;111(8):4658-702. doi: 10.1021/cr1004524. Epub 2011 Apr 22. Chem Rev. 2011. PMID: 21513269 Review. No abstract available.
References
-
- Archibald S.J., Smith R. Protein-Binding Metal Complexes: Noncovalent and Coordinative Interactions. In: Reedijk J., Poeppelmeier K., editors. Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications. Volume 3. Elsevier; Calgary, AB, Canada: 2013. pp. 661–682.
-
- Alsaedi S., Babgi B.A., Abdellatif M.H., Emwas A.-H., Jaremko M., Humphrey M.G., Hussien M.A. Effect of Net Charge on DNA-Binding, Protein-Binding and Anticancer Properties of Copper(I) Phosphine-Diimine Complexes. J. Inorg. Organomet. Polym. 2021;31:3943–3952. doi: 10.1007/s10904-021-02063-5. - DOI
-
- Krošl I., Otković E., Nikšić-Franjić I., Colasson B., Reinaud O., Višnjevac A., Piantanida I. Impact of positive charge and ring-size on interactions of calixarenes with DNA, RNA and nucleotides. New J. Chem. 2022;46:6860–6869. doi: 10.1039/D2NJ00061J. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

