Regenerative Inflammation: The Mechanism Explained from the Perspective of Buffy-Coat Protagonism and Macrophage Polarization
- PMID: 39457111
- PMCID: PMC11508762
- DOI: 10.3390/ijms252011329
Regenerative Inflammation: The Mechanism Explained from the Perspective of Buffy-Coat Protagonism and Macrophage Polarization
Abstract
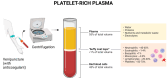
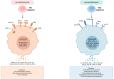
The buffy-coat, a layer of leukocytes and platelets obtained from peripheral blood centrifugation, plays a crucial role in tissue regeneration and the modulation of inflammatory responses. This article explores the mechanisms of regenerative inflammation, highlighting the critical role of the buffy-coat in influencing macrophage polarization and its therapeutic potential. Macrophage polarization into M1 and M2 subtypes is pivotal in balancing inflammation and tissue repair, with M1 macrophages driving pro-inflammatory responses and M2 macrophages promoting tissue healing and regeneration. The buffy-coat's rich composition of progenitor cells, cytokines, and growth factors-such as interleukin-10, transforming growth factor-β, and monocyte colony-stimulating factor-supports the transition from M1 to M2 macrophages, enhancing tissue repair and the resolution of inflammation. This dynamic interaction between buffy-coat components and macrophages opens new avenues for therapeutic strategies aimed at improving tissue regeneration and managing inflammatory conditions, particularly in musculoskeletal diseases such as osteoarthritis. Furthermore, the use of buffy-coat-derived therapies in conjunction with other regenerative modalities, such as platelet-rich plasma, holds promise for more effective clinical outcomes.
Keywords: buffy-coat; macrophage polarization; mesenchymal stem cells; platelet-rich plasma; regenerative inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sadri B., Hassanzadeh M., Bagherifard A., Mohammadi J., Alikhani M., Moeinabadi-Bidgoli K., Madani H., Diaz-Solano D., Karimi S., Mehrazmay M., et al. Cartilage Regeneration and Inflammation Modulation in Knee Osteoarthritis Following Injection of Allogeneic Adipose-Derived Mesenchymal Stromal Cells: A Phase II, Triple-Blinded, Placebo Controlled, Randomized Trial. Stem. Cell Res. Ther. 2023;14:162. doi: 10.1186/s13287-023-03359-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

