Clinical and Genetic Profiles of 5q- and Non-5q-Spinal Muscular Atrophy Diseases in Pediatric Patients
- PMID: 39457418
- PMCID: PMC11506990
- DOI: 10.3390/genes15101294
Clinical and Genetic Profiles of 5q- and Non-5q-Spinal Muscular Atrophy Diseases in Pediatric Patients
Abstract
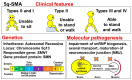
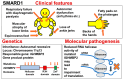
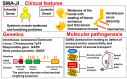
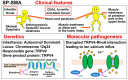
Background: Spinal muscular atrophy (SMA) is a genetic disease characterized by loss of motor neurons in the spinal cord and lower brainstem. The term "SMA" usually refers to the most common form, 5q-SMA, which is caused by biallelic mutations in SMN1 (located on chromosome 5q13). However, long before the discovery of SMN1, it was known that other forms of SMA existed. Therefore, SMA is currently divided into two groups: 5q-SMA and non-5q-SMA. This is a simple and practical classification, and therapeutic drugs have only been developed for 5q-SMA (nusinersen, onasemnogene abeparvovec, risdiplam) and not for non-5q-SMA disease.
Methods: We conducted a non-systematic critical review to identify the characteristics of each SMA disease.
Results: Many of the non-5q-SMA diseases have similar symptoms, making DNA analysis of patients essential for accurate diagnosis. Currently, genetic analysis technology using next-generation sequencers is rapidly advancing, opening up the possibility of elucidating the pathology and treating non-5q-SMA.
Conclusion: Based on accurate diagnosis and a deeper understanding of the pathology of each disease, treatments for non-5q-SMA diseases may be developed in the near future.
Keywords: 5q-SMA; SMA-JI; SMA-LED1; SMA-LED2; SMA-PME; SMARD1; SP-SMA; XL-SMA; non-5q-SMA.
Conflict of interest statement
H.N. reports personal compensation from Biogen Japan, Novartis Japan, and Chugai Pharmaceutical Co., and a consulting fee from Sekisui Medical Co. K.O. received personal compensation from Biogen Japan and Chugai Pharmaceutical Co. T.S. reports personal compensation from Biogen Japan, and Chugai Pharmaceutical Co. T.L. reports personal compensation from Chugai Pharmaceutical Co. and Novartis Japan. Y.T. reports personal compensation from Biogen Japan, Chugai Pharmaceutical Co., and Novartis Japan, and grant support from Novartis Japan. H.A. reports personal compensation from Biogen Japan, Chugai Pharmaceutical Co., and Novartis Japan, and grant support from Novartis Japan. The companies had no role in the design, execution, interpretation, or writing of the study. The other co-authors (E.T.E.N. and P.-S.L.) declare no competing interests.
Figures




References
-
- Dangouloff T., Vrščaj E., Servais L., Osredkar D., Adoukonou T., Aryani O., Barisic N., Bashiri F., Bastaki L., Benitto A., et al. Newborn Screening Programs for Spinal Muscular Atrophy Worldwide: Where We Stand and Where to Go. Neuromuscul. Disord. 2021;31:574–582. doi: 10.1016/j.nmd.2021.03.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

