Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study
- PMID: 39457420
- PMCID: PMC11507197
- DOI: 10.3390/genes15101296
Expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO in Relation to Left Ventricular Remodeling in Patients Six Months after the First Myocardial Infarction: A Prospective Study
Abstract
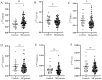
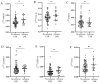
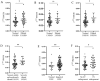
Background: After myocardial infarction (MI), adverse left ventricular (LV) remodeling may occur. This is followed by LV hypertrophy and eventually heart failure. The remodeling process is complex and goes through multiple phases. The aim of this study was to investigate the expression of HMGB1, TGF-β1, BIRC3, ADAM17, CDKN1A, and FTO, each involved in a specific step of LV remodeling, in association with the change in the echocardiographic parameters of LV structure and function used to assess the LV remodeling process in the peripheral blood mononuclear cells (PBMCs) of patients six months after the first MI. The expression of selected genes was also determined in PBMCs of controls. Methods: The study group consisted of 99 MI patients, who were prospectively followed-up for 6 months, and 25 controls. Cardiac parameters, measured via conventional 2D echocardiography, were evaluated at two time points: 3-5 days and 6 months after MI. The mRNA expression six-months-post-MI was detected using TaqMan® technology (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Results:HMGB1 mRNA was significantly higher in patients with adverse LV remodeling six-months-post-MI than in patients without adverse LV remodeling (p = 0.04). HMGB1 mRNA was significantly upregulated in patients with dilated LV end-diastolic diameter (LVEDD) (p = 0.03); dilated LV end-diastolic volume index (LVEDVi) (p = 0.03); severely dilated LV end-systolic volume index (LVESVi) (p = 0.006); impaired LV ejection fraction (LVEF) (p = 0.01); and LV enlargement (p = 0.03). It was also significantly upregulated in PBMCs from patients compared to controls (p = 0.005). TGF-β1 and BIRC3 mRNA were significantly lower in patients compared to controls (p = 0.02 and p = 0.05, respectively). Conclusions: Our results suggest that HMGB1 is involved in adverse LV remodeling six-months-post-MI, even on the mRNA level. Further research and validation are needed.
Keywords: ADAM17; BIRC3; CDKN1A; FTO; HMGB1; TGF-β1; gene expression; left ventricular remodeling; mRNA; myocardial infarction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bulluck H., Go Y.Y., Crimi G., Ludman A.J., Rosmini S., Abdel-Gadir A., Bhuva A.N., Treibel T.A., Fontana M., Pica S., et al. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017;19:26. doi: 10.1186/s12968-017-0343-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

