G6PD Potenza: A Novel Pathogenic Variant Broadening the Mutational Landscape in the Italian Population
- PMID: 39457422
- PMCID: PMC11507564
- DOI: 10.3390/genes15101298
G6PD Potenza: A Novel Pathogenic Variant Broadening the Mutational Landscape in the Italian Population
Abstract
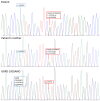
Background: Glucose 6 phosphate dehydrogenase (G6PD) is a rate-limiting enzyme of the pentose phosphate pathway. The loss of G6PD activity in red blood cells increases the risk of acute haemolytic anaemia under oxidative stress induced by infections, some medications, or fava beans. More than 200 single missense mutations are known in the G6PD gene. A 41-year-old woman with a family history of favism coming from the Basilicata region (Italy) was evaluated at our hospital for G6PD abnormalities. Methods: DNA was extracted from a peripheral blood sample and genotyped for the most common G6PD pathogenic variants (PVs). Positive results obtained by Restriction Fragment Length Polymorphism (RFLP), as per practice in our laboratory, were then reconfirmed in Sanger sequencing. Results:RFLP analysis highlighted a variant compatible with the G6PD Cassano variant. Confirmatory testing by Sanger unexpectedly identified a novel variant: c.1357G>A, p.(Val453Met) (NM_001360016.2); the same variant was found in the patient's mother. In silico models predicted a deleterious effect of this variant at the protein level. The novel G6PD variant was named "G6PD Potenza" on the basis of the patient's regional origin. Conclusions: This case describes a novel G6PD variant. It also highlights how the Sanger sequencing technique still represents an indispensable confirmatory standard method for variants that could be misinterpreted by only using a "first-level" approach, such as the RFLP. We stress that the evaluation of clinical manifestations in G6PD-deficient patients is of primary importance for the classification of each new G6PD mutation, in agreement with the new WHO guidelines.
Keywords: G6PD deficiency; Potenza; Sanger sequencing; acute haemolytic anaemia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Gómez-Manzo S., Marcial-Quino J., Vanoye-Carlo A., Serrano-Posada H., Ortega-Cuellar D., González-Valdez A., Castillo-Rodríguez R.A., Hernández-Ochoa B., Sierra-Palacios E., Rodríguez-Bustamante E., et al. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. Int. J. Mol. Sci. 2016;17:2069. doi: 10.3390/ijms17122069. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

