Genetics of Calcific Aortic Stenosis: A Systematic Review
- PMID: 39457433
- PMCID: PMC11508093
- DOI: 10.3390/genes15101309
Genetics of Calcific Aortic Stenosis: A Systematic Review
Abstract
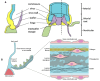
Background: Calcific aortic stenosis is the most prevalent valvular abnormality in the Western world. Factors commonly associated with calcific aortic stenosis include advanced age, male sex, hypertension, diabetes and impaired renal function. This review synthesises the existing literature on genetic associations with calcific aortic stenosis. Methods: A systematic search was conducted in the PubMed, Ovid and Cochrane libraries from inception to 21 July 2024 to identify human studies investigating the genetic factors involved in calcific aortic stenosis. From an initial pool of 1392 articles, 78 were selected for full-text review and 31 were included in the final qualitative synthesis. The risk of bias in these studies was assessed using the Newcastle Ottawa Scale. Results: Multiple genes have been associated with calcific aortic stenosis. These genes are involved in different biological pathways, including the lipid metabolism pathway (PLA, LDL, APO, PCSK9, Lp-PLA2, PONS1), the inflammatory pathway (IL-6, IL-10), the calcification pathway (PALMD, TEX41) and the endocrine pathway (PTH, VIT D, RUNX2, CACNA1C, ALPL). Additional genes such as NOTCH1, NAV1 and FADS1/2 influence different pathways. Mechanistically, these genes may promote a pro-inflammatory and pro-calcific environment in the aortic valve itself, leading to increased osteoblastic activity and subsequent calcific degeneration of the valve. Conclusions: Numerous genetic associations contribute to calcific aortic stenosis. Recognition of these associations can enhance risk stratification for individuals and their first-degree relatives, facilitate family screening, and importantly, pave the way for targeted therapeutic interventions focusing on the identified genetic factors. Understanding these genetic factors can also lead to gene therapy to prevent calcific aortic stenosis in the future.
Keywords: aortic stenosis; calcific.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Yadgir S., Johnson C.O., Aboyans V., Adebayo O.M., Adedoyin R.A., Afarideh M., Alahdab F., Alashi A., Alipour V., Arabloo J., et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation. 2020;141:1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

