Sleep Abnormalities in SLC13A5 Citrate Transporter Disorder
- PMID: 39457462
- PMCID: PMC11507356
- DOI: 10.3390/genes15101338
Sleep Abnormalities in SLC13A5 Citrate Transporter Disorder
Abstract
Background: SLC13A5 Citrate Transporter Disorder is a rare pediatric neurodevelopmental disorder. Patients have epilepsy, developmental disability, and impaired mobility. While sleep disorders are common in children with neurodevelopmental disorders, sleep abnormalities have not been reported in SLC13A5 patients.
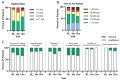
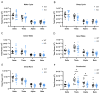
Methods: Here, we assessed sleep disturbances in patients through caregiver reported surveys and in a transgenic mouse model of SLC13A5 deficiency. A total of 26 patients were evaluated with the Sleep Disturbance Scale for Children three times over a one-year span. Sleep and wake activities were assessed in the SLC13A5 knock-out (KO) mice using wireless telemetry devices.
Results: A high burden of clinically significant sleep disturbances were reported in the patients, with heterogeneous symptoms that remained stable across time. While sleep disturbances were common, less than 30% of patients were prescribed medications for sleep. Comparatively, in SLC13A5 KO mice using EEG recordings, significant alterations were found during light cycles, when rodents typically sleep. During the sleep period, SLC13A5 mice had increased activity, decreased paradoxical sleep, and changes in absolute power spectral density, indicating altered sleep architecture in the mouse model.
Conclusions: Our results demonstrate a significant component of sleep disturbances in SLC13A5 patients and mice, highlighting a potential gap in patient care. Further investigation of sleep dysfunction and the underlying etiologies of sleep disturbances in SLC13A5 citrate transporter disorder is warranted.
Keywords: EEG; SDSC; SLC13A5; mice; patients; sleep.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Thevenon J., Milh M., Feillet F., St-Onge J., Duffourd Y., Juge C., Roubertie A., Heron D., Mignot C., Raffo E., et al. Mutations in SLC13A5 cause autosomal-recessive epileptic encephalopathy with seizure onset in the first days of life. Am. J. Hum. Genet. 2014;95:113–120. doi: 10.1016/j.ajhg.2014.06.006. - DOI - PMC - PubMed
-
- Gopal E., Miyauchi S., Martin P.M., Ananth S., Srinivas S.R., Smith S.B., Prasad P.D., Ganapathy V. Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G402–G408. doi: 10.1152/ajpgi.00371.2006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

