Recent Progress of Antisense Oligonucleotide Therapy for Superoxide-Dismutase-1-Mutated Amyotrophic Lateral Sclerosis: Focus on Tofersen
- PMID: 39457466
- PMCID: PMC11507444
- DOI: 10.3390/genes15101342
Recent Progress of Antisense Oligonucleotide Therapy for Superoxide-Dismutase-1-Mutated Amyotrophic Lateral Sclerosis: Focus on Tofersen
Abstract
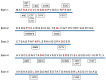
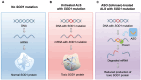
Amyotrophic lateral sclerosis (ALS) is a refractory neurodegenerative disease characterized by the degeneration and loss of motor neurons, typically resulting in death within five years of onset. There have been few effective treatments, making the development of robust therapies an urgent challenge. Genetic mutations have been identified as contributors to ALS, with mutations in superoxide dismutase 1 (SOD1), which neutralizes the harmful reactive oxygen species superoxide, accounting for approximately 2% of all ALS cases. To counteract the toxic gain of function caused by SOD1 mutations, therapeutic strategies aimed at suppressing SOD1 gene expression have shown promise. Antisense oligonucleotide (ASO) is an artificially synthesized, short, single-stranded DNA/RNA molecule that binds to target RNA to alter gene expression, representing a next-generation therapeutic approach. In 2023, tofersen became the first ASO drug approved by the FDA for ALS. Administered intrathecally, tofersen specifically binds to SOD1 mRNA, inhibiting the production of toxic SOD1 protein, thereby improving biomarkers of ALS. The long-term efficacy and safety of tofersen require further validation, and the development of more optimized treatment protocols is essential. A series of studies and therapeutic developments related to SOD1 mutations have advanced the understanding of ALS pathophysiology and significantly contributed to treatment strategies for central nervous system disorders. This review focuses on an overview of SOD1 mutations and the development process of tofersen, aiming to deepen the understanding of advancements in ALS research and discuss future challenges and directions for ASO therapy.
Keywords: amyotrophic lateral sclerosis (ALS); antisense oligonucleotide (ASO); central nervous system (CNS); superoxide dismutase 1 (SOD1); tofersen.
Conflict of interest statement
T.Y. is a cofounder and shareholder of OligomicsTx Inc., which aims to commercialize antisense technology. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

