Resveratrol Effect on α-Lactalbumin Thermal Stability
- PMID: 39457489
- PMCID: PMC11504486
- DOI: 10.3390/biomedicines12102176
Resveratrol Effect on α-Lactalbumin Thermal Stability
Abstract
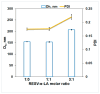
The effect of resveratrol (RESV) on α-lactalbumin (α-LA) thermal stability was evaluated using differential scanning calorimetry (DSC), circular dichroism (CD) and dynamic light scattering (DLS) measurements. Complementary information offered by molecular docking served to identify the binding site of the ligand on the native structure of protein and the type of interacting forces. DSC thermograms revealed a double-endotherm pattern with partial overlapping of the two components. The most relevant effect of RESV is manifested in the narrowing of the protein thermal fingerprint: the first process (peak temperature T1) is shifted to higher temperatures while the second one (peak temperature T2) to lower values. The CD data indicated partial conformational changes in the protein non-α-helix domain at T1, resulting in a β-sheet richer intermediate (BSRI) with an unaffected, native-like α-helix backbone. The RESV influence on this process may be defined as slightly demoting, at least within DSC conditions (linear heating rate of 1 K min-1). On further heating, unfolding of the α-helix domain takes place at T2, with RESV acting as a promoter of the process. Long time incubation at 333 K produced the same type of BSRI: no significant effect of RESV on the secondary structure content was detected by CD spectroscopy. Nevertheless, the size distribution of the protein population obtained from DLS measurements revealed the free (non-bound) RESV action manifested in the developing of larger size aggregates.
Keywords: CD; DSC; aggregates; lactalbumin; resveratrol; unfolding.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Brew K. α-Lactalbumin. In: McSweeney P.L.H., Fox P.F., editors. Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects. 4th ed. Springer; New York, NY, USA: 2013. pp. 261–273. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

