Exploring Liraglutide in Lithium-Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior
- PMID: 39457518
- PMCID: PMC11505538
- DOI: 10.3390/biomedicines12102205
Exploring Liraglutide in Lithium-Pilocarpine-Induced Temporal Lobe Epilepsy Model in Rats: Impact on Inflammation, Mitochondrial Function, and Behavior
Abstract
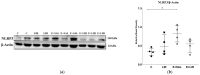
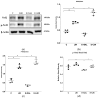
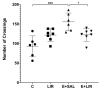
Background/Objectives: Glucagon-like peptide-1 receptor agonists such as liraglutide are known for their neuroprotective effects in neurodegenerative disorders, but their role in temporal lobe epilepsy (TLE) remains unclear. We aimed to investigate the effects of liraglutide on several biological processes, including inflammation, antioxidant defense mechanisms, mitochondrial dynamics, and function, as well as cognitive and behavioral changes in the TLE model. Methods: Low-dose, repeated intraperitoneal injections of lithium chloride-pilocarpine hydrochloride were used to induce status epilepticus (SE) in order to develop TLE in rats. Fifty-six male Sprague Dawley rats were subjected and allocated to the groups. The effects of liraglutide on inflammatory markers (NLRP3, Caspase-1, and IL-1β), antioxidant pathways (Nrf-2 and p-Nrf-2), and mitochondrial dynamics proteins (Pink1, Mfn2, and Drp1) were evaluated in hippocampal tissues via a Western blot. Mitochondrial function in peripheral blood mononuclear cells (PBMCs) was examined using flow cytometry. Cognitive-behavioral outcomes were assessed using the open-field, elevated plus maze, and Morris water maze tests. Results: Our results showed that liraglutide modulates NLRP3-mediated inflammation, reduces oxidative stress, and triggers antioxidative pathways through Nrf2 in SE-induced rats. Moreover, liraglutide treatment restored Pink1, Mfn2, and Drp1 levels in SE-induced rats. Liraglutide treatment also altered the mitochondrial function of PBMCs in both healthy and epileptic rats. This suggests that treatment can modulate mitochondrial dynamics and functions in the brain and periphery. Furthermore, in the behavioral aspect, liraglutide reversed the movement-enhancing effect of epilepsy. Conclusions: This research underscores the potential of GLP-1RAs as a possibly promising therapeutic strategy for TLE.
Keywords: antioxidant pathways; behavior; epilepsy; inflammation; liraglutide; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

