Potent Biological Activity of Fluorinated Derivatives of 2-Deoxy-d-Glucose in a Glioblastoma Model
- PMID: 39457553
- PMCID: PMC11504489
- DOI: 10.3390/biomedicines12102240
Potent Biological Activity of Fluorinated Derivatives of 2-Deoxy-d-Glucose in a Glioblastoma Model
Abstract
Background: One defining feature of various aggressive cancers, including glioblastoma multiforme (GBM), is glycolysis upregulation, making its inhibition a promising therapeutic approach. One promising compound is 2-deoxy-d-glucose (2-DG), a d-glucose analog with high clinical potential due to its ability to inhibit glycolysis. Upon uptake, 2-DG is phosphorylated by hexokinase to 2-DG-6-phosphate, which inhibits hexokinase and downstream glycolytic enzymes. Unfortunately, therapeutic use of 2-DG is limited by poor pharmacokinetics, suppressing its efficacy.
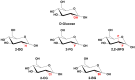
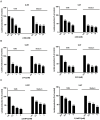
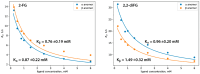
Methods: To address these issues, we synthesized novel halogenated 2-DG analogs (2-FG, 2,2-diFG, 2-CG, and 2-BG) and evaluated their glycolytic inhibition in GBM cells. Our in vitro and computational studies suggest that these derivatives modulate hexokinase activity differently.
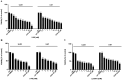
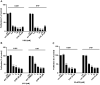
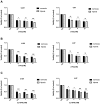
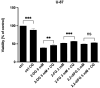
Results: Fluorinated compounds show the most potent cytotoxic effects, indicated by the lowest IC50 values. These effects were more pronounced in hypoxic conditions. 19F NMR experiments and molecular docking confirmed that fluorinated derivatives bind hexokinase comparably to glucose. Enzymatic assays demonstrated that all halogenated derivatives are more effective HKII inhibitors than 2-DG, particularly through their 6-phosphates. By modifying the C-2 position with halogens, these compounds may overcome the poor pharmacokinetics of 2-DG. The modifications seem to enhance the stability and uptake of the compounds, making them effective at lower doses and over prolonged periods.
Conclusions: This research has the potential to reshape the treatment landscape for GBM and possibly other cancers by offering a more targeted, effective, and metabolically focused therapeutic approach. The application of halogenated 2-DG analogs represents a promising advancement in cancer metabolism-targeted therapies, with the potential to overcome current treatment limitations.
Keywords: 2-deoxy-d-glucose; NMR spectroscopy; cytotoxic action; glycolysis; glycolysis inhibition; halogenated derivatives; hexokinase activity; molecular docking.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. W. Priebe is an inventor of patents covering new derivatives of 2-DG. He is the chair of SAB and a shareholder of Moleculin Biotech. Inc., and WPD Pharmaceuticals. His research is in part supported by a sponsor research grant from Moleculin Biotech. Inc. I. Fokt and R. Zielinski are listed as inventors on patents covering new analogs of 2-DG and are consultants of Moleculin Biotech., Inc., and are shareholders of Moleculin Biotech, Inc. Beata Pająk-Tarnacka is the CSO at WPD Pharmaceuticals. The other authors declare no conflicts of interest.
Figures

Similar articles
-
Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing "hypoxic tumor cells".Cancer Chemother Pharmacol. 2006 Dec;58(6):725-34. doi: 10.1007/s00280-006-0207-8. Epub 2006 Mar 23. Cancer Chemother Pharmacol. 2006. PMID: 16555088
-
WP1234-A Novel Anticancer Agent with Bifunctional Activity in a Glioblastoma Model.Biomedicines. 2022 Nov 3;10(11):2799. doi: 10.3390/biomedicines10112799. Biomedicines. 2022. PMID: 36359318 Free PMC article.
-
2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents.Int J Mol Sci. 2019 Dec 29;21(1):234. doi: 10.3390/ijms21010234. Int J Mol Sci. 2019. PMID: 31905745 Free PMC article. Review.
-
Synergistic Anticancer Effect of Glycolysis and Histone Deacetylases Inhibitors in a Glioblastoma Model.Biomedicines. 2021 Nov 23;9(12):1749. doi: 10.3390/biomedicines9121749. Biomedicines. 2021. PMID: 34944565 Free PMC article.
-
Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells.Antioxid Redox Signal. 2007 Sep;9(9):1383-90. doi: 10.1089/ars.2007.1714. Antioxid Redox Signal. 2007. PMID: 17627467 Review.
References
-
- Vlassenko A.G., McConathy J., Couture L.E., Su Y., Massoumzadeh P., Leeds H.S., Chicoine M.R., Tran D.D., Huang J., Dahiya S., et al. Aerobic glycolysis as a marker of tumor aggressiveness: Preliminary data in high grade human brain tumors. Dis. Markers. 2015;2015:874904. doi: 10.1155/2015/874904. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

