Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer's Disease In Vitro Model
- PMID: 39457556
- PMCID: PMC11504366
- DOI: 10.3390/biomedicines12102243
Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer's Disease In Vitro Model
Abstract
Background: Alzheimer's disease (AD) develops as a result of oxidative damage to neurons and chronic inflammation of microglia. These processes can be influenced by the use of a conditioned medium (CM) derived from mesenchymal stem cells (MSCs). The CM contains a wide range of factors that have neurotrophic, antioxidant, and anti-inflammatory effects. In addition, the therapeutic potential of the CM can be further enhanced by pretreating the MSCs to increase their paracrine activity. The current study aimed to investigate the neuroprotective effects of CM derived from MSCs, which were either activated by a TLR3 ligand or exposed to CoCl2, a hypoxia mimetic (pCM or hCM, respectively), in an in vitro model of AD.
Methods: We have developed a novel in vitro model of AD that allows us to investigate the neuroprotective and anti-inflammatory effects of MSCs on induced neurodegeneration in the PC12 cell line and the activation of microglia using THP-1 cells.
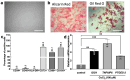
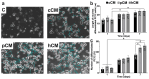
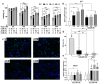
Results: This study demonstrates for the first time that pCM and hCM exhibit more pronounced immunosuppressive effects on proinflammatory M1 macrophages compared to CM derived from untreated MSCs (cCM). This may help prevent the development of neuroinflammation by balancing the M1 and M2 microglial phenotypes via the decreased secretion of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and increased secretion of IL-4, as well as the expression of IL-10 and TGF-β by macrophages. Moreover, a previously unknown increase in the neurotrophic properties of hCM was discovered, which led to an increase in the viability of neuron-like PC12 cells under H2O2-induced oxidative-stress conditions. These results are likely associated with an increase in the production of growth factors, including vascular endothelial growth factor (VEGF). In addition, the neuroprotective effects of CM from preconditioned MSCs are also mediated by the activation of the Nrf2/ARE pathway in PC12 cells.
Conclusions: TLR3 activation in MSCs leads to more potent immunosuppressive effects of the CM against pro-inflammatory M1 macrophages, while the use of hCM led to increased neurotrophic effects after H2O2-induced damage to neuronal cells. These results are of interest for the potential treatment of AD with CM from preactivated MSCs.
Keywords: Alzheimer’s disease; MSCs; PC12; immunosuppression; microglia; neuroprotection; secretome.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Yokokawa K., Iwahara N., Hisahara S., Emoto M.C., Saito T., Suzuki H., Manabe T., Matsumura A., Matsushita T., Suzuki S., et al. Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress. J. Alzheimer’s Dis. 2019;72:867–884. doi: 10.3233/JAD-190817. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

