Evaluation of Biochemical Serum Markers for the Diagnosis of Polycystic Ovary Syndrome (PCOS) in Obese Women in Kazakhstan: Is Anti-Müllerian Hormone a Potential Marker?
- PMID: 39457645
- PMCID: PMC11504444
- DOI: 10.3390/biomedicines12102333
Evaluation of Biochemical Serum Markers for the Diagnosis of Polycystic Ovary Syndrome (PCOS) in Obese Women in Kazakhstan: Is Anti-Müllerian Hormone a Potential Marker?
Abstract
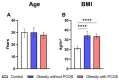
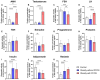
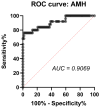
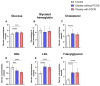
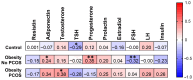
Background: Polycystic Ovarian Syndrome (PCOS) is a common endocrine condition that affects 8-13% of women of reproductive age. In Kazakhstan, the prevalence of this syndrome is particularly high compared with other countries and the global average. Currently, the diagnosis of PCOS is based on internationally established Rotterdam criteria, using hyperandrogenism as a key parameter. These criteria are applied to diagnose PCOS in all female patients, although obese patients may have excess testosterone produced by adipose tissue. To avoid possible misdiagnosis, an additional criterion, especially for the diagnosis of PCOS in obese women, could be considered. The aim of this study was to identify whether anti-Müllerian hormone (AMH) or other biochemical criteria can be used for this purpose. Methods: A total of 138 women were recruited for this study and grouped into control (n = 46), obese subjects without PCOS (n = 67), and obese patients with PCOS (n = 25). The health status, anthropometric parameters, and serum indicators for glucose, glycosylated hemoglobin, and hormone levels were examined for all subjects. Statistical data were analyzed using GraphPad Prism 10 software for interpretation of the data. Results: Serum AMH, testosterone, and LH were positively correlated in obese PCOS patients, while AMH and FSH were negatively correlated. Compared with other biochemical indicators, the serum AMH and testosterone levels in obese PCOS patients were significantly higher than those in non-PCOS patients (regardless of obesity), and AMH was also positively correlated with testosterone. Conclusions: AMH appears to be a reliable criterion in addition to testosterone for the diagnosis of PCOS in obese women.
Keywords: anti-Müllerian hormone (AMH); biomarker; hyperandrogenism; obesity; polycystic ovary syndrome (PCOS).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Miazgowski T., Martopullo I., Widecka J., Miazgowski B., Brodowska A. National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: The modeled estimates from the Global Burden of Disease Study 2016. Arch. Med. Sci. 2021;17:343–351. doi: 10.5114/aoms.2019.87112. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

